Md Ashraf Mokhtar1, Mohd Aftab Ahmad1, Qudsia Nizami1, Farhan Jalees Ahmad2, Md Mahfoozur Rahman3
1Department of Pharmacology, Faculty of Medicine, Jamia Hamdard, New Delhi-110062, India.
2Department of Pharmaceutics, Faculty of Pharmacy, Jamia Hamdard, New Delhi-110062, India.
3Department of Pharmacology, Nizamia Unani Medical College, Dumri, via Buniad Ganj, Rasoolpur , Gaya-823001, India.
ORIGINAL RESEARCH ARTICLE
Volume 2, Issue 1, Jan-April 2014.
Article history
Received: 22 March 2014
Revised: 10 April 2014
Accepted: 23 April 2014
Early view: 28 April 2014
*Author for correspondence
E-mail: [email protected]
Mobile/ Tel.: 0000000000
Keywords:
Incision wound
Excision wound
Tensile strength
Framycetin
Epithelialization.
Objective: The aim of the present study was to verify the wound healing claims of a Unani formulation Marham-e-Ral on different experimental models of wounds in albino rats.
Material and methods: Marham-e-Ral was prepared as per classical method. The animals were divided into three groups of six each. The parameters studied were wound contraction, epithelialisation period and tensile strength.
Results: Marham-e-Ral showed wound healing activity, with almost 98.9% healing on 15th day, whereas in the control only 90% healing took place. In the incision wound studies, there was a significant increase in tensile strength on day 10 due to treatment with standard cream (31.90 + 0.026) and Marham-e-Ral (16.48 ± 0.022) when compared with control group i.e. 8.388 + 0.128 (P ≤ 0.01). The histopathological slides clearly showed the extent of healing and demonstrated significant improvement.
Conclusion: Marham-e-Ral showed a wholesome effect on wound healing in excision wound model and the effect was found equally comparable to the standard drug framycetin cream.
INTRODUCTION
One of the most important compound formulations of Unani system is Marham-e-Ral. It is used as wound healer and also effective in various skin lesions like boils, syphilitic ulcer and fistula, etc. (Khan, 1996; Said, 1997; Anonymous, 1986).
The literature survey revealed that the ingredients of Marham-e-Ral contain triterpenoids and chalcone glycoside in Shorea robusta (Anonymous, 1995; Jain et al., 1982), glycerides and flavonols in Cinnamomum camphora (Kafoor) (Mukherjee et al., 1994), catechin, tannins, glycoside and quercetin in Acacia catechu (Katha), (Azad, 2001; Trease and Evans, 2002; Yadav & Sodhi, 2002), esters and alcohols in Beeswax (Jackson, 2006; Tulloch, 1971), and volatile acids & glycerides in Ghee (Prasad & Dorle, 2006). Several pharmacological studies of Marham-e-Ral have been reported viz. Shorea robusta posses anti-inflammatory and anti-bacterial activity (Das et al., 2003). Cinnamomum camphora has anti-inflammatory action due to modulation of cytokine, NO & prostaglandin E2 (Lee et al., 2006) and antifungal activity (Srivastava et al., 2008). Acacia catechu showed antioxidant (Naik et al., 2005) and antimicrobial activity (Singh et al., 2005). Beeswax posses antiulcer activity (Carbajal, 1995) and Ghee has wound healing activity (Charde et al., 2006; Prasad & Dorle, 2006). On the basis of above mentioned references Marham-e-Ral was chosen for the evaluation of wound healing potential in rat models.
MATERIAL AND METHODS
Marham-e-Ral
Ingredients of Marham-e-Ral are as follows: Ral (Oleo Resin of Shorea robusta)-50 g, Kafoor (Solid ketone of Cinnamomum camphora)-50 g, Katha (Extract of Acacia catechu)-50 g, Roghan-e-Gao (Clarified butter of cow)-200 g, Wax (Bees wax)-50 g, (Said, 1997; Anonymous, 1986).
Plant materials
Ral (Oleo Resin of Shorea robusta), Kafoor (Solid ketone of Cinnamomum camphora) and Katha (Extract of Acacia catechu) were purchased from Green Earth Pvt. Ltd., New Delhi, India. Beeswax was purchased from Khari Bawli, Delhi, India and Ghee was obtained from Amul outlet, New Delhi, India. The authenticity and identity of the drugs were confirmed on the basis of classical description in Unani literature at Department of Ilmul Advia, Faculty of Medicine (Unani) and modern botanical information was established by Department of Botany, Faculty of Science, Jamia Hamdard, New Delhi, India. Standard used was Framycetin sulphate 1% cream (Aventis) (Manjunatha, et al., 2005).
Preparation of Marham-e-Ral
Marham-e-Ral was prepared as per classical method described in Unani literature (Said, 1997; Khan, 1996; Anonymous, 1993; Anonymous, 1986). Ral (Shorea robusta), and Katha (Acacia catechu) were finely ground and sieved through 100 number mesh after which the camphor was first triturated with little and later the whole safoof. The ghee and wax were melted and powdered mixture of katha, ral & kafoor was added to the ghee-wax mixture, stirred till both mixed well and stocked in a glass jar.
Experimental animal
Healthy male/female inbred albino rats of Wistar strain of 4-6 months; weighing 150-200 g were used in the present study. They were procured from Central Animal House Facility, Jamia Hamdard and were housed and maintained at standard housing. They were fed with a commercial diet and water ad libitum during experiment. Approval for experimental studies was obtained from Institutional Animal Ethical Committee, Jamia Hamdard, Hamdard Nagar, New Delhi, India.
Excision wound model
The rats were fasted overnight and were inflicted with excision wounds (Morton & Malone, 1972). Animals were anaesthetized by open mask method with anesthetic ether and their back were shaved with depilatory and cleaned with dry gauze piece. Rats were depilated on the back and a circular wound of about 500 mm2 full thickness were excised in the dorsal inter scapular region (Mukherjee et al., 2000). Full aseptic measures had not been taken and no local or systemic antimicrobials were used throughout the experiment. The animals were divided into three groups (control, standard and Marham-e-Ral) of six animals each. The creams were topically applied once a day, starting from the day of operation, till complete epithelialization. The parameters studied were wound contraction, epithelialization period.
Measurement of contraction and epithelialization of wound
Contraction which mainly contributes for wound closure was studied by tracing the raw wound area on transparent paper on day 3, 6, 9, 12, and 15th and thereafter every alternate day till wounds were completely covered with epithelium (Manjunatha et al., 2005). These wound tracing were retraced on a millimeter scale graph paper to determine the wound area. On each measurement day the wounds of the animals were photographically documented. Wound contraction (WC) was calculated as a percentage change in the initial wound size i.e.
WC(%)= Initial wound size–specific day wound size X 100
Initial wound size
Epithelialization period was monitored by noting the number of days required for eschar to fall away, leaving no raw wound area behind (Kamath, et al., 2006).
Incision wound model
The animals were anesthetized with intra peritoneal injection of pentobarbitone in the dose of 50 mg/kg; and their back were shaved with depilatory and cleaned with dry gauze piece, and a 6 cm long para-vertebral incision of the skin on either side of the vertebral column of the rat was made (Ehrlich et al., 1968). The wounds were made 1 cm away laterally to the vertebral column, after hemostasis, wounds were cleaned with sterile wet cotton and then closed with interrupted sutures of 1cm apart using a surgical thread (no. 000) and a curved needle (no. 11) (Swamy et al., 2007; Lodhi et al., 2006; Singh et al., 2006). Immediately after operation, the rats were placed in the collars to prevent damage to the wounds. Full aseptic measures had not been taken and no local or systemic antimicrobials were used throughout the experiment. The animals were divided into 3 groups (control, standard and Marham-e-Ral) of 6 animals each. The creams were topically applied once in a day. On the 9th day after wounding sutures were removed and the tensile strength was measured on the 10th day (Mukherjee et al., 2000; Singh et al., 2006).
Measurement of tensile strength of the wound
Tensile strength is the resistance to breaking under tension. It indicates how much the repaired tissue resists to breaking under tension and may indicate in part the quality of the repaired tissue. For this purpose the newly repaired tissue including scar was excised to measure the tensile strength (Rashed et al., 2003). Tensile strength of wounds was determined in all three groups on day 10 by texture analyzer (TA XT2). The rats were anesthetized with light ether. The skin was removed with 1 cm on each side of the wound. Tensile strength was measured using a texture analyzer (TA XT2) and the increase in tensile strength served as a measure of wound healing. Tensile strength was determined using the following equations (Saringat & Sheikh, 2000).
Tensile strength = Breaking load (force)/Area
Area = Thickness × Width
Statistical analysis
All the values were expressed as mean ± SEM. The statistical significance was determined by one-way ANOVA followed by Dunnett test. Values P < 0.05 and P < 0.01 were considered as significant and P < 0.001 as highly significant.
RESULTS
Excision wound test
The mean percentage closure of wound area was calculated on 3rd, 6th, 9th, 12th, 15th and 18th post wounding day. The observations are shown in the table 1 & 2 and fig. 1-8. In control group wound contracted to the extent of 90.3% by day 15 on the other hand Marham-e-Ral showed wound contraction 98.93% which is significant (P < 0.01), to control group. The period of epithelialization of wounds were found to be faster i.e. 16.00 ± 0.36 day in Marham-e-Ral group as compared to the animals of control group where it was found 21.33±0.21 day. While period of epithelialization was 15.33 ± 0.21 day in the standard group. The differences in mean epithelialization of wounds treated by Marham-e-Ral and compared with control groups was found significant (P < 0.01).
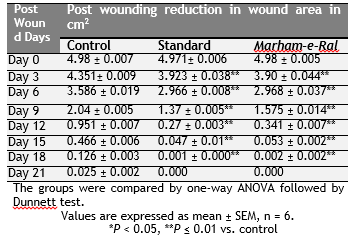 |
Table. 1 Effect of Marham-e-Ral on post wounding reduction in wound area in excision wound model. Click here to view full image |
 |
Table. 1 Effect of test formulations on wound contraction & period of epithelialization in excision wound model. Click here to view full image |
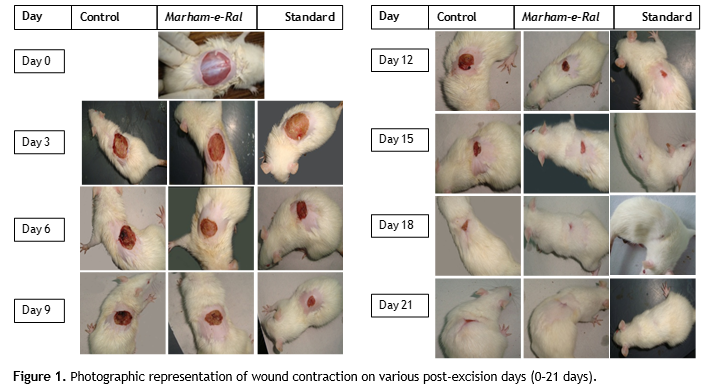 |
Figure.1 Photographic representation of wound contraction on various post-excision days (0–21 days). Click here to view full image |
Incision wound test
In incision wound model, the mean breaking strength of 10 days old wound was significantly increased in the animal group treated with standard drug framycetin 1% w/w cream i.e. 31.90 ± 0.026 n/cm2 (P < 0.01). The mean breaking strength in Marham-e-Ral group was measured as 16.48 ± 0.022 (P < 0.01) and was found significant (P < 0.01), when compared with the control group (8.388 ± 0.128). The observations are shown in the Table 3.
 |
Table. 3 Effect of test formulations on breaking strength in incision wound model. Click here to view full image |
DISCUSSION
Herbal medicines in wound management involve disinfection, debridement and providing a moist environment to encourage the establishment of the suitable environment for natural healing process (Priya et al., 2002). The results in table 1-3 indicate that the Marham-e-Ral have wound healing activity, with 98.9% healing on 15th day, similar to standard (99.05%), where as in the control only 90 % healing took place on 15th day. The effects produced by test ointment were comparatively lesser than standard but produced better response in comparison to control group. The literature survey revealed that the ingredients of Marham-e-Ral has been screened scientifically for its efficiency by various researchers and has been reported to contain a number of phytochemicals, which are responsible for its various important pharmacological actions (Mukherjee et al., 1994; Azad, 2001; Trease and Evans, 2002; Yadav & Sodhi, 2002).
The present study found the anti-inflammatory activity of Marham-e-Ral in Wistar albino rats, representing different phases of inflammation. Wound healing is stepwise process, which comprises of various phases such as hemostasis, inflammation, proliferative and remodeling or maturation. The genetic reaction regulating the body’s own cellular resistance mechanisms plays to the wound and its repair (Charles et al., 1995). Therefore, in the present study, excision and incision wound models were kept to assess the effect of Marham-e-Ral on different sphases. In incision wound, enhance intensile strength of treated wounds may be because of enhance in collagen concentration and stabilization of the fibers (Udupa et al., 1995).
The results showed that Marham-e-Ral possesses a marked prohealing stroke demonstrated by a significant enhance in the rate of wound contraction and by increased epithelialization period. Recent studies with other plant extracts have revealed that phytochemical constituents like flavanoids (Tsuchiya et al., 1996), triterpenoids (Scortichini et al., 1991) and tannins (Rane et al., 2003) are known to encourage the wound-healing process. Preliminary phytochemical screening constituents of Marham-e-Ral exhibited the presence of alkaloids, flavonoids and tannins (Mukherjee et al., 1994; Azad, 2001; Trease and Evans, 2002; Yadav & Sodhi, 2002). Presence of these ingredients may have promoted the wound healing process mainly due to their astringent and antimicrobial property, which seems to be responsible for wound contraction and increased rate of epithelialisation. The analgesic and anti-inflammatory effects prove to be of added advantage in such conditions.
CONCLUSION
It can be concluded that the Marham-e-Ral is able to potentiate the natural healing process, reducing the time period of onset of healing and the completion of healing process until the epithelialisation took place. It is obvious that pain and inflammation will be relieved earlier than the natural healing time.
ACKNOWLEDGEMENTS
The authors are very grateful to Faculty of Medicine, Jamia Hamdard, New Delhi, India for providing necessary facilities to carry out the present study; and Scientific & Digital System, New Delhi, India for their help in handling Texture Analyzer (TA XT2).
CONFLICT OF INTEREST
None declared.
REFERENCES
Anonymous. National Formulary of Unani medicine, 1st edition (Urdu). Ministry of Health & Family Welfare, Government of India: New Delhi, India, 1993.
Anonymous. Qarabadeen-e Majeedi, 9th edition. Ajanta Officiate Packaging Pvt Ltd: Delhi, India, 1986.
Anonymous. The Wealth of India, IX vol. Publication and Information Directorate CSIR: New Delhi, India, 1995.
Azad AK, Ogiyama K, Sassa T. Isolation of (+)-catechin and a new polyphenolic compound in bengal catechu. J Wood Sci. 47, 406-409, 2001.
Carbajal D, Molina V, Valdes S, Arruzazabala L, Mas R. Anti-ulcer activity of higher primary alcohols of beeswax. J Pharm Pharmacol. 47, 731-733, 1995.
Charde MS, Fulzele SV Satturwar PM, Joshi SB, Kasture AV. Wound healing and antiinflammatory potential of Madhu ghrita. Indian J Pharm Sci. 68, 26-31, 2006.
Charles VM, Rusell RCG, Williams NS. Short Practice of Surgery, 20th edition. Champan and Hall: London, 1995.
Das DJ, Rath CC, Ganapathy S, Narayana KL, Swamy KM, Bapuji M. Effect of ultrasound on antibacterial activity of pyrolysed sal (Shorea robusta Gaertn) resin. Indian Perfumer. 47, 189, 2003.
Ehrlich HP, Hunt TK. Effect of cortisone and vitamin A on wound healing. Ann Surg. 164, 324-8, 1968.
Jackson MA & Eller FJ. Isolation of long-chain aliphatic alcohols from beeswax using lipase-catalyzed methanolysis in supercritical carbon dioxide. J Supercritical Fluids. 37, 173-177, 2006.
Jain A, Bhartiya HP, Vishwakarma AN. A chalcone glycoside from the heartwood of Shorea robusta. Phytochemistry. 21, 957, 1982.
Kamath S, Rao SG, Murthy KD, Bairy KL, Bhat S. Enhanced wound contraction and epithelization period in steroid treated rats: Role of pyramid environment. Indian J Exp Biol. 44, 902-904, 2006.
Khan A. Qarabadeen-e-Azam. Ejaz Publishing House: New Delhi, India, 1996.
Lee HJ, Hyuna EA, Yoon WJ, Kim BH, Rhee MH, Kang HK, Cho JY, Yoo ES. In vitro anti-inflammatory and anti-oxidative effects of Cinnamomum camphora extracts. J Ethnopharmacol. 103, 208–216, 2006.
Lodhi S, Pawar RS, Jain AP, Singhai AK. Wound healing potential of Tephrosia purpurea (Linn.) Pers. in rats. J Ethnopharmacol. 108, 204–210, 2006.
Manjunatha BK, Vidya SM, Rashmi KV, Mankani KL, Shilpa HJ, Singh SDJ. Evaluation of wound-healing potency of Vernonia arborea Hk. Idian J Pharmacol. 37, 223-226, 2005.
Morton JJ, Malone MH. Evaluation of vulnerary activity by an open wound procedure in rats. Arch Int Pharmacodyn Ther. 196, 117-126, 1972.
Mukherjee PK, Verpoorte R, Suresh B. Evaluation of in-vivo wound healing activity of Hypericum patulum leaf extract on different wound model in rats. J Ethnopharmacol. 70, 315-321, 2000.
Mukherjee RK, Fujimoto Y, Kakinuma K. 1-(ω-Hydroxyfattyacyl) glycerols and two flavanols from Cinnamomum camphora. Phytochemistry. 3, 1641-1643, 1994.
Naik GH, Priyadarsini KI, Mohan H. Evaluating the antioxidant activity of different plant extracts and herbal formulations. Res Chem Intermed. 31, 145-151, 2005.
Prasad V, Dorle AK. Evaluation of ghee based formulation for wound healing activity. J Ethnopharmacol. 107, 38–47, 2006.
Priya KS, Gnanamani A, Radhakrishnan N, Babu M. Healing potential of Datura alba on burn wounds in albino rats. J Ethnopharmacol. 83, 193-199, 2002.
Rane, M, Madhura, Mengi, Shusma, A. Comparative effect of oral admin- istration and topical application of alcoholic extract of Terminalia arjuna bark on incision and excision wounds in rats. Fitoterapia. 74, 553–558, 2003.
Rashed AN, Afifia FU, Disi AM. Simple evaluation of the wound healing activity of a crude extract of Portulaca oleracea L. (growing in Jordan) in Mus musculus JVI-1. J Ethnopharmacol. 88, 133-136, 2003.
Said M. Hamdard Pharmacopoeia of Eastern Medicine. Sri Sat Guru Publication: New Delhi, India, 1997.
Saringat HB, Sheikh KA. The wound healing properties of Channa striatus-cetrimide cream—tensile strength measurement. J Ethnopharmacol. 71, 93–100, 2000.
Scortichini, M, Pia, Rossi MJ. Applied Bacteriol. 71, 109, 1991.
Singh M, Govindarajan R, Nath V, Rawat AKS, Mehrotra S. Antimicrobial, wound healing and antioxidant activity of Plagiochasma appendiculatum Lehm. et Lind. J Ethnopharmacol. 107, 67–72, 2006.
Singh R, Jain A, Panwar S, Gupta D, Khare SK. Antimicrobial activity of some natural dyes. Dyes Pigm. 66, 99-102, 2005.
Srivastava B, Singh P, Shukla R, Dubey NK. A novel combination of the essential oils of Cinnamomum camphora and Alpinia galangal in checking aflatoxin B1 production by a toxigenic strain of Aspergillus flavus. World J Microbiol Biotech. 24, 693-695, 2008.
Swamy HMK, Krishna V, Shankarmurthy K, Rahiman BA, Mankani KL, Mahadevan KM., Harish BG, Naika HR. Wound healing activity of embelin isolated from the ethanol extract of leaves of Embelia ribes Burm. J Ethnopharmacol. 109, 529–534, 2007.
Trease, GE, Evans WC, 2002. Pharmacognosy, 15th edition. Saunders Publishers: London, 2002.
Tsuchiya H, SatoM, Miyazaki T, Fujiwara S, Tanigaki S, Ohyama M, Tanaka T, Iinuma M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J Ethnopharmacol. 50, 27–34, 1996.
Tulloch AP. Beeswax: Structure of the esters and their component hydroxy acids and diols. Chem Phys Llipids. 6, 235-265, 1971.
Udupa AL, Kulkarni DR, Udupa SL. Effectof Tridax procumbans extracts on woundhealing. Int J Pharmacog. 33, 37–40, 1995.
Yadav RN, Sodhi S. A new flavone glycoside: 5,7,3′,4′-tetrahydroxy-3-methoxy flavone-7-o-g-d-galactopyranosyl-(1m4)-o-g-d-glucopyranoside from the Stem of Acacia catechu Willd. Journal of Asian Natural Products Research, 4, 11 -15, 2002.









 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.