Ansari JA1*, Abdul Mateen Syed2, Md Faruque Ahmad3, Mirza Anwar Baig4
1Department of Pharmacology, MESCO College of Pharmacy, Mustaidpura, Karwan Road, Hyderabad-500006, TS, India.
2Department of Pharmacology, Oriental College of Pharmacy, Sector-2, Sanpada (W), Navi Mumbai-400705, India.
3College of Applied Medical Sciences, Jazan University, Jazan, KSA.
4School of Pharmacy, Anjuman-I-Islam’s, Kalsekar Technical Campus, Panvel-410206, MS, India.
ORIGINAL RESEARCH ARTICLE
Volume 2, Issue 3, Page 142-147, September-December 2014.
Article history
Received: 10 November 2014
Revised: 30 November 2014
Accepted: 15 December 2014
Early view: 30 December 2014
*Author for correspondence
E-mail: [email protected]
Background: The leaves of Aegle marmelos (L.) Correa (AM) is widely used by tribal people of Chidambaram district (Tamilnadu, India) for the treatment of Jaundice and other liver diseases. Aim of the present study was to evaluate the medicinal potential ethanolic extract of AM as hepatoprotective and antioxidant against acetaminophen-induced liver damage.
Material and methods: The hepatoprotective activity of ethanol extract of AM was evaluated by acetaminophen-induced liver damage and antioxidant activity was evaluated using in vivo antioxidant models viz. lipid peroxidation assay and glutathione estimation in liver. Liver function tests were measured to detect hepatoprotective activity, which was further supported by histopathological examination.
Results: Ethanol extract of AM reduced elevated level of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and bilirubin significantly which was comparable to standard drug Silymarin in acetaminophen treated Wistar rats (P < 0.01). Further, ethanol extract of AM was found to reduce the elevated levels of lipid peroxidation product malondialdehyde (MDA), and enhance the reduced level of glutathione in liver proving antioxidant activity comparable with Silymarin.
Conclusion: Aegle marmelos (L.) Correa posses hepatoprotective property and may be effective in oxidative stress-induced cholestatic hepatic injury.
Keywords: Antioxidant, Hepatoprotective; Glutathione; Histopathology; Lipid Peroxidation.
INTRODUCTION
The usage of plants, plant extracts or plant-derived pure chemicals to treat disease become a therapeutic modality, which has become the test of time. According to the World Health Organization (WHO), about three-quarters of the world population depends upon traditional remedies (mainly herbs) for the health care of its people (Ansari et al., 2013; Ansari and Inamdar, 2010). India has a rich heritage of traditional knowledge and is platform to several important time-honored systems of health care such as Unani, Ayurveda and Siddha. It has been expected that the proportion of medicinal plants in India (7,500 of the 17,000 higher plant species are medicinal plants) is higher than any country of the world with respect to the existing flora of that respective country (Ansari and Rashid, 2012; Shiva, 1996). Within the European market, herbal medicines represent an important pharmaceutical market with annual sales of US $7 billion. Modern medicines have little to offer for alleviation of hepatic diseases and it is chiefly the plant based preparations which are employed for the treatment of liver disorders (Arumugam et al., 2008). The potential of these medicinal plants must be proved so as to identify newer medicaments acting against these disorders. In view of this, the present study was aimed at evaluating the hepatoprotective activity of the leaves of Aegle marmelos (L.) Correa against acetaminophen-induced hepatotoxicity in rats.
Aegle marmelos (L.) Correa (AM), (Rutaceae) is a popular medicinal plant in the Ayurvedic and Siddha systems of medicine and folk medicines used to curb a wide variety of ailments. The plant, commonly known as the bael tree, is native to the Indo-Malayan region and is currently cultivated in India, Pakistan, Bangladesh, Sri Lanka, Burma, and Thailand (Arumugam et al., 2008). The leaves of AM are astringent, laxative, febrifuge, expectorant and are useful in ophthalmia, deafness, inflammations, catarrh, diabetes, ulcers, dropsy, cholera and beri-beri associated with a weakness of the heart and asthmatic complaints (Nadkarni, 1976). Aqueous leaf extract and methanol extract of the root bark of AM showed preventive effects on myocardial diseases. The aqueous extract of leaf possesses a hypoglycemic effect. Essential oil isolated from the leaf has antifungal activity (Arumugam et al., 2008). AM leaf extract was found to be a potential antioxidant drug, which decreases the blood sugar level in alloxan induced diabetic rats (Sabu and Ramadasan, 2004). It was found to be as effective as insulin in the restoration of blood glucose and body weight to normal levels on hyperglycemic state (Seema et al., 1996). The ethanol extract of AM leaf possesses anti spermatogenic activity (Sur et al., 1999) and aqueous extract of the leaf has anti motility action on spermatozoa in rats (Sur et al., 2002). The roots and bark of tree are used in the treatment of fever by making a decoction significant against malaria. The ripe fruit is a good cure for diabetes, dyspepsia, constipation and body heating problems (Mishra et al., 2010).
MATERIALS AND METHODS
Reagents
Acetaminophen, Silymarin were purchased from M/s Sigma Chemicals, USA. Carboxymethyl cellulose sodium salt (CMC) was purchased from G.S. Chemical Testing Lab and Allied Industries, Bombay, India and was used for preparing acetaminophen suspension. All other chemicals used were of analytical grade and were purchased from E. Merck, Germany and India; Sigma Chemicals Co., St. Louis, USA and SRL Chemicals, India.
Plant collection and extraction
Fresh leaves of AM were collected locally from Chidambaram during the month of June. The identification of Aegle marmelos was carried out by Prof. Dr. R. Selvaraj, Chief Botanist, Department of Botany, Annamalai University, Annamalai Nagar Chidambaram, Cuddalore, Tamil Nadu, India. A voucher specimen has been kept at the herbarium of the University. Fresh leaves were dried under shade until a constant weight was obtained, powdered with a mechanical grinder and pass through sieve no 40. The sieved powder was stored in airtight container and keep in room temperature for the further study. The powdered leaves (500 g) were defatted with petroleum ether (60–80 ◦C) dried marc obtained was subjected to successive soxhlet extraction for about 48 h. The solvents were removed from the extracts under reduced pressure by using rotary vacuum evaporator. The percentage yield of ethanol extract was 15.95 % (w/w) from the starting raw material. The dried mass was diluted with normal saline and used in experiments.
Phytochemical analysis
Qualitative phytochemical analysis of the extract was carried out for assaying presence of carbohydrates, glycosides, proteins, amino acids, phytosterols, saponins, flavonoids, alkaloids and tannins (Kokate, 1990).
Experimental animals
Female albino Wistar rats (150–200 g) were used throughout the experimental study. The animals were housed in polypropylene cages with sterile inert husk materials as bedding. The experimental animals were maintained under controlled environment condition at 23 ± 2°C and relative humidity 55 ± 10% with a 12 h dark: light cycle. They were allowed to acclimatize for one week and were provided a free access to standard pellet diet (Gold Mohur, Hindustan Lever Ltd., Mumbai, India) and water ad libitum. The experimental protocol was approved by the institutional animal ethical committee of MESCO College of Pharmacy, Hyderabad, India.
Acetaminophen-induced liver damage
Acetaminophen was suspended in 1 % CMC and administered p.o., at a dose of 2.5 g/kg. This dose is known to cause liver damage in rats (Mitra et al., 1998). Animals were divided into five groups of 6 animals each. The first group (control) received saline (0.9%NaCl) 1 ml/kg for six days. The group II received ethanol extract of AM 500 mg/kg for six days. The groups III, IV and V received 1 % CMC in distilled water, ethanol extract of AM (500 mg/kg, p.o.) and Silymarin (100 mg/kg p.o.) respectively once a day for six days. On the fourth day, 30 min after the administration of the respective treatments, all the animals of groups III, IV and V were administered with acetaminophen (2.5 g/kg p.o.). On the sixth day after 2 h of respective treatments, under ether anesthesia, blood samples were collected in centrifuge tubes via retro orbital route, and the serum was separated. The abdomen was then cut open and liver samples were removed. In order to prevent RBC contamination, samples were cut into small slices, rinsed thoroughly in ice cold normal saline and blotted dry with blotting paper.
Serum samples were frozen immediately in a deep freezer at –20 °C and enzyme assays were performed on the next day. After washing with ice-cold normal saline tissue samples were transferred to ice cold 0.02 M EDTA for estimation of glutathione sulfhydryl groups (GSH), and to 1.15% KCl for estimation of lipid peroxidation. The homogenized samples were taken immediately for estimation.
Biochemical analysis
Enzyme assays
The blood plasma was separated after centrifuging the blood sample at 5000 rpm for 5 min and the following biochemical metabolites of blood plasma and enzymes were quantified: the activities of aspartate aminotransferase; AST and alanine aminotransferase; ALT (Reitman and Frankel, 1957), total bilirubin (Malloy and Evelyn, 1937), alkaline phosphatase (ALP) (Kind and King,1954) and total proteins (Henry, 1964).
Lipid peroxidation
In vivo quantification of lipid peroxidation was performed using the spectrophotometric method of Ohkawa et al. (1979). The lipid peroxidation product malondialdehyde (MDA) was measured with the help of a standard curve obtained by plotting absorbance against varying concentrations of 1, 1, 3, 3-tetraethoxypropane (TEP) in the range of 0 to 40 nmoles. The results are expressed as nmoles of MDA/g of tissue (wet wt).
Estimation of reduced glutathione (GSH)
Reduced glutathione (GSH) was estimated by its reaction with dithio-bis-2-nitrobenzoic acid (DTNB) that gives a yellow coloured complex with absorption maximum at 412 nm (Moron et al., 1979).
Histopathological examination
Liver tissue slices were fixed in 10% formalin. The hepatocytes profiles were assessed by making paraffin section slides of the liver. 4 µm paraffin sections were stained with haematoxylin and eosin (H&E). Five fields were viewed for histopathological signs at a magnification of 400. Events of central nuclear disintegration, central necrosis, Kuppfer cell hyperplasia, sinusoidal dialation and increased cytoplasmic eosinophilia were graded as the following (-): negative findings; (+) evidence of pathological changes; (++) mild pathological changes; (+++) moderate pathological changes; and (++++) marked pathological changes.
Statistical analysis
The obtained raw data in each experimental group were computed into mean and standard error of mean (SEM). Significance test (t-test) was computed between the control and treated groups.
RESULTS
The decoction contained carbohydrates, glycosides, amino acids, proteins, tannins, flavanoids, and phytosterols.
Biochemical observations
Acetaminophen treatment induced significant increase in the activities of plasma AST, ALT, total bilirubin, direct bilirubin, alkaline phosphatase and MDA were significantly elevated after acetaminophen treatment, whereas the total protein and GSH were significantly reduced as a result of acetaminophen treatment (Table 1). The mixed treatment of AM and acetaminophen induced non-significant changes of the levels of AST, ALT, ALP total bilirubin, direct bilirubin, total protein, MDA and GSH compared with control rats while acetaminophen alone treated animals showed significant changes. It was found that the levels of AST, ALT, ALP total bilirubin, direct bilirubin, total protein, MDA and GSH did not significantly differ between rats treated with the mixture of Silymarin and acetaminophen and control rats (Table 1-2).
 |
Table1. Changes in biochemical parameters of control and treated rats for 6 days. Click here to view full image |
 |
Table 2. Changes in biochemical parameters of control and treated rats for 6 days. Click here to view full image |
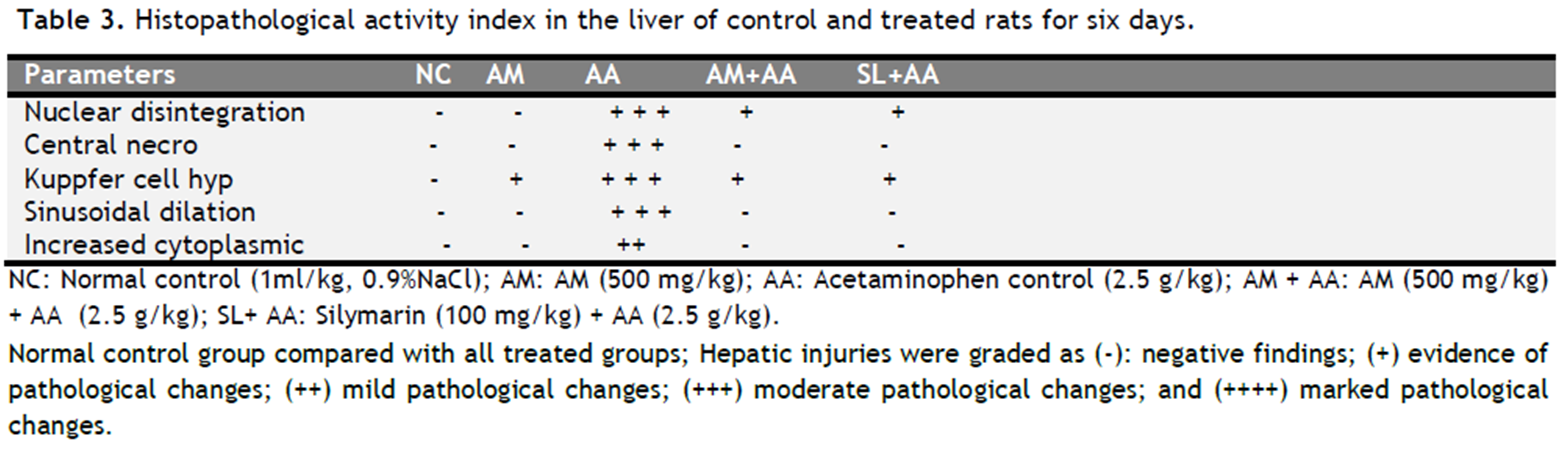 |
Table 3. Histopathological activity index in the liver of control and treated rats for six days. Click here to view full image |
Histopathological observations
Healthy control rats showed no histopathological changes in the liver (Fig. 1A and Table 3), whereas, treatment with acetaminophen showed central nuclear disintegration, central necrosis, Kuppfer cell hyperplasia, sinusoidal dialation and increased cytoplasmic eosinophilia (Fig. 1C and Table 3). The liver histopathology of rat treated with AM along with acetaminophen or Silymarin along with acetaminophen showed few degenerated cells (Fig. 1D & 1E, and Table 3). The liver histopathology of rat treated with AM alone showed a normal profile (Fig. 1B and Table 3).
By examining the histological activity index (HAI, Table 3) of the liver, it was found that rats challenged with acetaminophen showed moderate pathological changes (central nuclear disintegration, central necrosis, Kuppfer cell hyperplasia, and sinusoidal dialation) and mild increased cytoplasmic eosinophilia, zero histopathological signs were recorded for both control and AM treated rats. The combined treatment of AM along with acetaminophen and Silymarin along with acetaminophen showed few disintegrated cells and Kuppfer cell hyperplasia which are significantly different from the control group. Thus, the mutual treatment of AM and acetaminophen significantly reduces the histopathological effects (Fig. 1D & 1E and Table 3) which is identical to marketed hepatoprotective preparation Silymarin.
 |
Figure 1. Fig. 1A; Photograph of liver histopathology of normal control rats (H &E × 400); normal lobular architecture and normal hepatic cells; Fig. 1B; Photograph of rat liver treated with AM (H&E 400·) showed no significant morphological changes, as compared to animals in the control group; Fig. 1C; Photograph of rat liver treated with acetaminophen (H&E 400·) showing moderate pathological changes (central nuclear disintegration, central necrosis, Kuppfer cell hyperplasia, and sinusoidal dialation) and mild increased cytoplasmic eosinophilia; Fig. 1D; Photograph of rat liver treated with AM and acetaminophen (H&E 400·) showing few disintegrated cells and Kuppfer cell hyperplasia; Fig. 1E; Photograph of rat liver treated with Silymarin and acetaminophen (H&E 400·) showing same changes as in AM and acetaminophen animals i.e. few disintegrated cells and Kuppfer cell hyperplasia.. Click here to view full image |
DISCUSSION
Drugs are an important reason of hepatotoxicity. In general more than 900 drugs, toxins, and herbs have been reported to cause hepatotoxicity, and drugs account for 20-40% of all instances of fulminant hepatic failure. Acute hepatitis, with or without cholestasis, is the usual histological pattern of DILI (drug-induced liver disease), and drugs such as acetaminophen are the leading causes of acute liver failure (Ansari, 2010). Acetaminophen is a common antipyretic agent which is safe in therapeutic doses but can produce fatal hepatic necrosis in man, rats and mice with toxic doses (Prescott et al., 1971; Mitchell et al., 1973). Acetaminophen is mainly metabolized in liver to excretable glucuronide and sulphate conjugates (Jollow et al., 1974; Wong et al., 1981). However, hepatotoxicity of acetaminophen has been attributed to the formation of toxic metabolites when a part of acetaminophen is activated by hepatic cytochrome P-450 (Savides and Oehme, 1983) to a highly reactive metabolite (NAPQI) N-acetyl-p-benzoquinoneimine (Vermeulen, et al., 1992). Due to liver injury, the transport function of the hepatocytes gets disturbed, resulting in the leakage of plasma membrane (Zimmerman and Seeff, 1970), thereby causing an increased enzyme level in the serum. Protection against acetaminophen induced toxicity has been used as a test for a potential hepatoprotective agent by several investigators (Singh and Handa, 1995).
Plasma AST, ALT and bilirubin are the most sensitive biomarkers employed in the diagnosis of liver diseases (Pari and Kumar, 2002). During hepatocellular damage, varieties of enzymes normally located on the cytosol are released into the blood flow. Their quantification in plasma is a useful biomarker of the extent and type of hepatocellular damage (Pari and Murugan, 2004). The increased activities of these biomarkers observed in the present study correspond to the extensive hepatic damage induced in rats treated with acetaminophen. This is confirmed from histopathological signs observed in the present investigation. This was previously recorded by Yen et al., (2008) in which a marked rise of transaminases, phosphatase and bilirubin were recorded in acetaminophen-treated rats.
Sulfhydryl compounds are among the most important endogenous antioxidants. Glutathione (GSH) is the main intracellular nonprotein sulfhydryl and it plays an important role in the maintenance of cellular proteins and lipids in their functional states. NAPQI binds to GSH, forming a conjugate which results in conversion of GSH to oxidized form of glutathione. When GSH is lowered, the toxic effects of oxidative insult are exacerbated, resulting in increased membrane and cell damage. At this point, other protein and non-protein sulfhydryl groups present in the cell provide an important alternate protection (Genet et al., 2000). Pretreatment with AM helped in maintaining the levels of GSH (Table 1).
Any oxidative insult to a cell induces lipid peroxidation of cell-membrane lipids. The toxic peroxidative products cause widespread damage of macromolecules (Sinclair et al., 1991). Malondialdehyde (MDA), a secondary product of lipid peroxidation, is widely used to assess lipid peroxidation in animal tissues. AM effectively prevented acetaminophen-induced lipid peroxidation in liver cells. Decreased MDA levels in animals who received only AM suggest that AM, per se, decreases ongoing lipid peroxidation in normal liver cells as well (Table 1). Elevated levels of these enzymes in serum in acetaminophen treated group point to liver dysfunction. Pretreatment with AM reduced these elevations significantly, showing that AM also maintains the functional capacity of the liver. The beneficial effect of AM on biochemical parameters is also indicated by histopathological observations. The regenerative activity seen in liver cells of animals treated with acetaminophen signifies the compensatory changes to cellular insult. Preservation of lobular architecture and less prominent regenerative activity in liver sections of animals pretreated with AM showed that it helps to maintain the structural integrity of liver against acetaminophen -induced damage (Figs. A to E and Table 3).
CONCLUSION
Based on our present findings, it can be concluded that Aegle marmelos (L.) Correa protects the liver from acetaminophen induced hepatic damage by acting as an antioxidant.
CONFLICT OF INTEREST
None declared.
REFERENCES
Ansari JA, Inamdar NN. The promise of traditional medicines. Int J Pharmacol. 6, 808-812, 2010.
Ansari JA, Rashid A. Hepatoprotective effect of Tabernaemontana divaricata L. against acetaminophen-induced liver toxicity. Medicin Chem Drug Disc. 3, 154-159, 2012.
Ansari JA, Sayyed M, Manavalan R, Balamurgan. Hepatoprotective effect of Tribulus terrestris L. against acetaminophen-induced liver damage in Wistar rats. Euroasian J Hepato-Gastroenterol. 3, 15-18, 2013.
Ansari JA. Therapeutic approaches in management of drug-induced hepatotoxicity. J Biol Sci. 10, 386-395, 2010.
Arumugam S, Kavimani S, Kadalmani B, Ahmed AB, Akbarsha MA, Rao MV. Antidiabetic activity of leaf and callus extracts of Aegle marmelos in rabbit. Sci Asia. 34, 317-321, 2008.
Genet S, Kale RK, Baquer NZ. Effects of free radicals on cytosolic creatine kinase activities and protection by antioxidant enzymes and sulfhydryl compounds. Mol Cell Biochem. 210, 23–28, 2000.
Henry RJ. Clinical Chemistry (Principles and Techniques). Harper & Row: New York, 1964.
Jollow DJ, Thorgeirsson SS, Potter WZ. Hashimoto M, Mitchell JR. Acetaminophen-induced hepatic necrosis. VI. Metabolic disposition of toxic and non-toxic doses of acetaminophen. Pharmacol. 12, 251–271.
Kind PRN, King EJ. Estimation of plasma phosphatase by determination of hydrolysed phenol with antipyrine. J Clin Pathol. 7, 322–330, 1954.
Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. 1st edition. Nirali Prakashan: Pune, India. 1990.
Malloy E, Evelyn K. The determination of bilirubin with the photoelectric colorimeter. J Biolog Chem. 119, 481–485, 1937.
Mishra BB, Desh D. Singh, Navneet Kishore, Vinod K. Tiwari, Vyasji Tripathi. Antifungal constituents isolated from the seeds of Aegle marmelos. Phytochem. 71, 230–234, 2010.
Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BN. Acetaminophan induced hepatic necrosis.1. Role of drug metabolism. J Pharmacol Exp Ther. 187, 185–194, 1973.
Mitra SK, Venkataranganna MV, Sundaram K, Gopumadhavan S. Protective effect of HD-03, a herbal formulation against various hepatotoxic agents in rats. J Ethnopharmacol. 63, 181–186, 1998.
Moron MS, Depierre JW, Mannervik B. Levels of glutathione. Glutathione reductase and glutathione-S-transferase activities in rat lung and liver. Biochem Biophys Acta. 582, 67–78, 1979.
Nadkarni AK. Indian Materia Medica, 3rd Edition. Popular Press: Mumbai, India. 1976.
Ohkawa H, Oshihi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95, 351–358, 1979.
Pari L Kumar AN. Hepatoprotective activity of Moringa oleifera on antitubercular drug induced liver damage in rats. J Medicine Food. 5, 171–177, 2002.
Pari L, Murugan P. Protective role of tetrahydrocurcumin against erythromycin estolate-induced hepatotoxicity. Pharmacol Res. 49, 481–486, 2004.
Prescott LF, Wright N, Roscoe P, Brown SS. Plasma paracetamol half life and hepatic necrosis in patients with paracetamol overdose. Lancet. 519–522, 1971.
Reitman S, Frankel AS. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. American J Clin Pathol. 28, 56–63, 1957.
Sabu MC, Ramadasan K. Antidiabetic activity of Aegle marmelos and its relationship with its antioxidant properties. Indian J Physiol Pharmacol. 48:81– 88, 2004.
Savides MC, Oehme FW. Acetaminophen and its toxicity. J Appl Toxicol. 3, 95–111, 1983.
Seema PV, Sudha B, Padayatti PS, Abraham A, Raghu KG, Paulose CS. Kinetic studies of purified malate dehydrogenase in liver of streptozotocin-diabetic rats and the effect of leaf extract of Aegle marmelose (L.) Correa ex Roxb. Indian J Exp Biol. 34, 600–602, 1996.
Shiva MP. Inventory of Forestry Resources for Sustainable Management and Biodiversity Conservation. Indus Publishing Company: New Delhi, India. 1996.
Sinclair AI, Barnett AH, Lennie J. Free radicals and antioxidant system in health and diseases. J Appl Med. 17, 409, 1991.
Singh A, Handa SS. Hepatoprotective activity of Apium graveolens and Hygrophila auriculata against paracetamol and thioacetamide intoxication in rats. J Ethnopharmacol. 49, 119–126, 1995.
Sur TK, Pandit S, Paramanik T, Bhattacharyya D. Effect of Aegle marmelos leaf on rat sperm motility: An in vitro study. Indian J Pharmacol. 34, 276 –277, 2002.
Vermeulen NPE, Bessems JGM, Van de Streat R. Molecular aspects of paracetamol induced hepatotoxicity and its mechanism based prevention. Drug Metab Rev. 24, 367–407, 1992.
Wong LT, Whitehouse LW, Solemonraj G, Paul CJ. Pathways of disposition of acetaminophen conjugate in the mouse. Toxicol Lett. 9, 145–151, 1981.
Yen FL, Wu TH, Lin LT, Cham TM, Lin CC. Nanoparticles formulation of Cuscuta chinensis prevents acetaminophen-induced hepatotoxicity in rats. Food Chem Toxicol. 46, 1771–1777, 2008.
Zimmerman HJ, Seeff LB. Enzymes in hepatic disease. In: Goodly, El. (Ed.), Diagnostic Enzymology. Lea & Febiger, Philadelphia, 1–38, 1970.









 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.