Lakshmi Bhupati VS*, Muvvala S, Shashank P
Department of Pharmacology, Malla Reddy College of Pharmacy, Dhulapally Maisammaguda, Secunderabad-500014, AP, India.
ORIGINAL RESEARCH ARTICLE
Volume 2, Issue 1, Jan-April 2014.
Article history
Received: 04 April 2014
Revised: 20 April 2014
Accepted: 26 April 2014
Early view: 28 April 2014
*Author for correspondence
E-mail: [email protected]
Mobile/ Tel.: +91 9885324334
Keywords:
Doxorubicin
Vitis vinifera
Heart
Liver
Kidney
Lipid peroxidation.
Background: : Doxorubicin (DXN) is the most active cytotoxic agents having efficacy in malignancies either alone or combined with other cytocidal agents. The clinical usefulness of the anthracycline drug has been precluded by oxidative stress. This study is based on the possible protective effect of hydroalcoholic extract of Vitis vinifera against DXN-induced oxidative stress in male Wistar albino rats.
Material and methods: : Total six groups of six animals each were used for the present study. Group I received normal saline 0.9% for 30 days, group II received saline for 30 days and a single dose of DXN (15mg/kg i.p.) on 28th day, group III received standard vitamin E (100 mg/kg p.o.) for 30 days and a single dose of DXN (15 mg/kg i.p.) on 28th day, groups IV-VI received extract (100, 200 & 300 mg/kg p.o., respectively) for 30 days and a single dose of DXN (15 mg/kg i.p.) on 28th day.
Results:
Administration of DXN produced marked biochemical alterations of oxidative stress including increased levels of various biochemical parameters like lactate dehydrogenase (LDH), troponin, serum glutamic-pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), alkaline phosphatase (ALP), serum creatinine, blood urea nitrogen (BUN), malonal dialdehyde (MDA) and decreased levels of total protein, antioxidant enzymes- superoxide dismutases (SOD), catalase, glutathione (GSH). But prior administration of hydroalcoholic extract of Vitis vinifera ahead of DXN challenge ameliorated all these biochemical markers.
Conclusion:
The results of the present study revealed the protective effect of hydroalcoholic extract of Vitis vinifera in DXN-induced oxidative stress in heart, liver and kidney.
INTRODUCTION
Doxorubicin (Adriamycin), an anthracycline antibiotic, is widely used in the treatment of a variety of human malignancies, including breast cancer, small cell carcinoma of the lung and acute leukemia’s (Blum and Carter, 1974). Like most of the anticancer drugs, DXN also causes various toxic effects, including cardiotoxicity which leads to acute and chronic heart failure (Koima et al., 1993), hepatotoxicity (Gillick et al., 2002) and nephrotoxicity (Yilmaz et al., 2006).
Cellular damage induced by DXN is mediated by the formation of an iron-anthracycline complex that generates free radicals, which, in turn, causes severe damage to the plasma membrane and interferes with the cytoskeletal structure (Billingham et al., 1978). Due to the presence of a less developed antioxidant defense mechanism, heart is particularly vulnerable to injury by anthracycline-induced reactive oxygen species.
Although the exact mechanism of DXN-induced nephrotoxicity remains unknown, it is believed that the toxicity may be mediated through free radical formation, iron-dependent oxidative damage of biological macromolecules, membrane LPO, and protein oxidation (Liu et al., 2007). DXN-induced changes in the kidneys of rats include increased glomerular capillary permeability and tubular atrophy (Wapstra et al., 1999).
Vitis vinifera (Black grape) is one of the most widely grown fruit crops in the world. Grape juice jams and raisins are also important commodities in the market of the whole world. Numerous studies focused on the health-promoting and antioxidant effects of grapes. Interest in the health benefits of muscadines has increased due to their high phenolics contents. Most phenolics in muscadines are located in the seeds (Poudel et al., 2008). Gallic acid, catechin and epicatechin are the main phenolics found in muscadine seeds, while ellagic acid and myricetin are the major ones in the skins. Muscadines and Grapes, well known for their high levels of antioxidants and polyphenols, have also shown promise as novel antimicrobial agents (Brown et al., 2009), anti-cancer properties (Mertens-Talcott et al., 2006), anti-inflammatory activity (Greenspan et al., 2006) and antimicrobial activity against Escherichia coli O157:H7 (Kim et al., 2009), antiulcerative, antiarthritic, anti-viral, prevent skin aging, scavenge free radicals and inhibit UV-radiation induced peroxidation activity (Bagachi et al., 1997; Dragsted et al., 1998).
Therapeutic strategies, designed to augment cellular endogenous defense systems have been identified as a promising approach to combat oxidative stress-associated disease conditions (Krishenbaum and Singal, 1993). The hypothesis proposed was that if DXN cardiotoxicity, hepatotoxicity and nephrotoxicity are related to free radical formation and oxidative stress, an antioxidant such as Vitis vinifera may protect against DXN-induced toxicity in the heart, liver and kidney.
In view of these facts the present study was designed to test the hypothesis whether a nutritional strategy like chronic administration of hydroalcoholic extract of Vitis vinifera could prevent DXN-induced cardiotoxicity, hepatotoxicity and nephrotoxicity in terms of oxidative stress.
MATERIAL AND METHODS
Collection of plant material
The black grapes were collected from the local market in Ranga Reddy District and the botanical authentication was done by Dr. Ram Chandra Reddy, Head of Department of Botany, Osmania University, Hyderabad, India.
Preparation of the hydroalcoholic extract
The skin, seed and pulp were separated from black grapes and were shade dried individually. The dried skin, seed and pulp were ground to powder. This dried powder was used for soxhlet extraction. Extraction was done by using the soxhlet apparatus at a temperature below 60 °C for 24 hours. Powder was extracted with 60% water and 40% methanol. The solvent thus obtained was evaporated under vaccum to get a semi-solid form of the extract. Percentage yield was 62.5% with respect to dried powder. Oral suspensions containing 100, 200 and 400 mg of extract were prepared by using distilled water which was used for evaluation of the activity.
Chemicals
Doxorubicin (Oncodria, Sun Pharmaceutical Ind. Ltd. Gujarat, India) was obtained from the local market. 1, 1-diphenyl, 2- picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich, Mumbai, India. All chemicals for sensitive biochemical assays were obtained from Sigma Chemicals Co. India and Hi media Chemicals, Mumbai, India. Distilled water was used for biochemical assays. All kits were obtained from Span Diagnostics Ltd., Surat, India.
Animals
Adult male Wistar albino rats (150 ± 10 g body weight) were obtained from the departmental animal facility where they were housed under standard husbandry conditions (25 ± 2 °C temp., 60–70% relative humidity and 12 h photoperiod) with standard rat feed and water ad libitum. Experiments were conducted in accordance with the guidelines set by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India and experimental protocols were approved by the Institutional Animal Ethics Committee (CPCSEA/1217/2008/a).
Experimental design
Thirty six Wistar albino male rats weighing 150-250 g were selected for this study. Animals were divided into six groups of 6 animals each:
Group I: Control i.e. normal control group (1 ml, 0.9% normal saline).
Group II: DXN i.e. negative control group (Intraperitoneal injection of DXN 15 mg/kg body weight).
Group III: VIT E i.e. positive control group (vitamin E 100 mg/kg body weight p.o. + DXN 15 mg/kg body weight i.p.).
Group IV: VVE 1 i.e. test group dose I (Vitis vinifera extract 100 mg/kg body weight p.o. + DXN 15 mg/kg body weight i.p.).
Group V: VVE 2 i.e. test group dose II (Vitis vinifera extract 200 mg/kg body weight p.o. + DXN 15 mg/kg body weight i.p.).
Group VI: VVE 3 i.e. test group dose III (Vitis vinifera extract 400 mg/kg body weight p.o. + DXN 15 mg/kg body weight i.p.
Animals were grouped into six groups as mentioned above. Group I i.e. normal control group animals were given 1 ml of 0.9% normal saline for 30 days. Group II animals (DXN; negative control group) were given normal saline until 30th day and a single dose of DXN 15 mg/kg body weight i.p. on 28th day. Group III (VIT E: positive control group) animals were given vitamin E 100 mg/kg body weight p.o. until 30th day and a single dose of DXN 15 mg/kg body weight i.p. on 28th day. Group IV-VI (VVE 1-3: test groups I-III) animals were given Vitis vinifera extract 100, 200 & 400 mg/kg body weight p.o. until 30th day and a single dose of DXN 15 mg/kg body weight i.p. on 28th day, respectively.
Serum sample preparation
The animals were sacrificed 48 h after the injection of DXN by using light ether anesthesia; blood was collected by cardiac puncture method. Blood was centrifuged using Remi cool centrifuge at 4000 rpm for 15 mins. Serum was separated for the estimation of various biochemical parameters like serum LDH (Henry et al., 1960), serum troponin (Melanson et al., 2007), SGPT and SGOT (Reitman and Frankel, 1957), alkaline phosphatase (Fiske and Subbarow, 1925), total protein and blood urea nitrogen (BUN) (Ohkawa et al., 1979) and serum creatinine (Bowers, 1980).
Tissue sample preparation
Heart, liver and kidney tissues were separated and washed with phosphate buffer saline (0.05 M, ph7.4). Then minced into small pieces and homogenized in ice cold phosphate buffer saline (0.05M, ph7.4) using tissue homogenizer to obtain 1:9 (w/v) (10%) whole homogenate. A part of the liver homogenate was taken and mixed with equal volume of 10% trichloroacetic acid (TCA) for the estimation of malondialdehyde. Homogenate was centrifuged using Remi cool centrifuge at 8000 rpm for 30 mins. The supernatant was separated and used for estimation of anti-oxidant levels of different enzymes i.e. Super oxide dismutase, catalase, glutathione and malondialdehyde in all the three tissues – heart, liver and kidney (Shiromwar and Chidrawar, 2011; Saber et al., 2011; Shinde et al., 2010).
Statistical analysis
The experimental results were expressed as the mean ± SEM with 6 rats in each group. The intergroup variation between various groups were analyzed statistically using one-way analysis of variance (ANOVA) using the Graph Pad Prism version 5.0, followed by Dunnett’s multiple comparison test (DMCT). Results were considered statistically significant when P < 0.05.
RESULTS
Effect of hydroalcoholic extract of Vitis vinifera on serum LDH
Animals treated with DXN produced a significant (P < 0.05) increase in the level of LDH as compared with the rats in control group. Pretreatment with standard Vitamin E 100 mg/kg and Vitis vinifera 400 mg/kg significantly (P < 0.05) decreased the levels of LDH with respect to the DXN treated group (Fig. 1).
Effect of hydroalcoholic extract of Vitis vinifera on serum troponin
For control group a distinct pinkish purple line in the control region ‘C’ appeared which indicates non-reactive for Troponin-I. For DXN treated group a purple coloured line was appeared in the test region ‘T’ and pinkish purple line in the control region ‘C’ which indicates increase in the levels of the Troponin-I when compared to the control group. Standard vitamin E 100 mg/kg and Vitis vinifera 400 mg/kg has shown only a distinct pinkish purple line in the control region’C’ and no color in the test region ‘T’ which indicates the significant decrease in levels of Troponin-I when compared to the DXN treated group.
Effect of hydroalcoholic extract of Vitis vinifera on serum SGPT, SGOT, alkaline phosphatase and total protein
Animals treated with DXN produced a significant (P < 0.05) increase in the level of SGPT, SGOT and ALP, decrease in total protein levels as compared with control group. Pretreatment with Vitis vinifera 400 mg/kg significantly decreased levels of SGPT (P < 0.001), SGOT (P < 0.01) and ALP (P < 0.001) as that of standard vitamin E level, whereas the value of total protein significantly increased (P < 0.001) as that of vitamin E when compared to DXN treated group (Table 1).
Effect of hydroalcoholic extract of Vitis vinifera on blood urea nitrogen (BUN) and serum creatinine
Animals treated with DXN produced a significant (P < 0.05) increase in serum creatinine and blood urea nitrogen when compared to that of control group. Pretreatment with Vitis vinifera 400 mg/kg significantly (P < 0.001) decreased serum creatinine and blood urea nitrogen as that of standard vitamin E when compared to that of DXN treated group (Table 2).
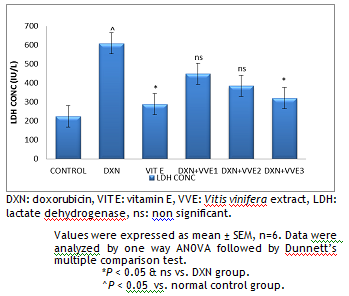 |
Figure 1. Effect of hydroalcoholic extract of Vitis vinifera on serum LDH. |
DXN: doxorubicin, VIT E: vitamin E, VVE: Vitis vinifera extract, LDH: lactate dehydrogenase, ns: non significant.
Values were expressed as mean ± SEM, n=6. Data were analyzed by one way ANOVA followed by Dunnett’s multiple comparison test.
*P < 0.05 & ns vs. DXN group.
^P < 0.05 vs. normal control group
Effect of hydroalcoholic extract of Vitis vinifera on cardiac GSH, catalase and SOD.
The GSH, SOD and catalase levels were significantly (P < 0.05) decreased in the DOX-treated group as compared to normal control animals. Pretreatment with standard vitamin E 100 mg/kg and Vitis vinifera 400 mg/kg showed a significant increase in GSH, SOD (P < 0.001) and catalase (P < 0.05) as compared to DXN treated group (Fig. 2). Treatment with Vitis vinifera 200 mg/kg showed a significant increase in GSH, SOD, and catalase (P < 0.05) when compared to that of DXN treated group.
Effect of hydroalcoholic extract of Vitis vinifera on hepatic SOD, GSH, catalase and lipid peroxidation (MDA).
The GSH, SOD and catalase levels were significantly (P < 0.05) decreased in the DXN-treated group as compared with normal control animals. Pretreatment with standard vitamin E 100 mg/kg and Vitis vinifera 400 mg/kg showed a significant increase in glutathione, superoxide dismutase (P < 0.001), catalase (P < 0.05) and significant decrease in MDA (P < 0.01) when compared to that of DXN treated group. Treatment with Vitis vinifera 200 mg/kg showed a significant increase in glutathione, superoxide dismutase, catalase (P < 0.05), significant decrease in MDA (P < 0.05) when compared to that of DXN treated group (Fig. 3, 4).
Effect of hydroalcoholic extract of Vitis vinifera on renal SOD, GSH and catalase
The GSH, SOD and catalase levels were significantly (P < 0.05) decreased in the DXN-treated group as compared with normal control animals. Pretreatment with standard vitamin E 100 mg/kg and Vitis vinifera 400 mg/kg showed a significant increase in GSH, SOD (P < 0.001) and catalase (P < 0.05) when compared to that of DXN treated group. Treatment with Vitis vinifera 200 mg/kg showed a significant increase in GSH, SOD and catalase (P < 0.05) when compared to that of DXN treated group (Fig. 4).
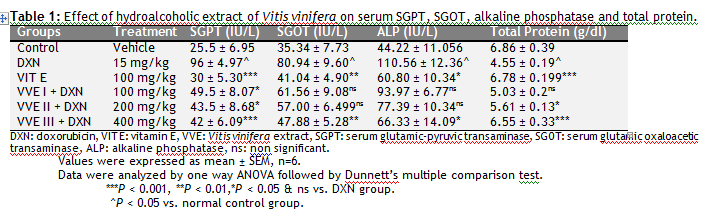 |
Table 1: Effect of hydroalcoholic extract of Vitis vinifera on serum SGPT, SGOT, alkaline phosphatase and total protein. |
 |
Table 1: Effect of hydroalcoholic extract of Vitis vinifera on BUN and serum creatinine. |
DISCUSSION
DXN is a broad spectrum antibiotics used as a chemotherapeutic drug for the treatment of different forms of human neoplastic disease (Hertzan-Levy et al., 2000). However, the clinical use of anticancer drug is greatly limited by its dose-dependent toxicity (Al-majed et al., 2002). Free radicals generation and lipid peroxidation have been suggested to be responsible for DXN-induced toxicity in different tissues including heart, liver and kidney (Chularojmontri et al., 2005; Xu al., 2001; Saber et al., 2011). This oxygen derived radicals’ causes severe damage to plasma membrane and interferes with cytoskeleton assembly (Uchiyama et al., 1978).
 |
Figure 2 (A & B). Effect of hydroalcoholic extract of Vitis vinifera on cardiac GSH, catalase and SOD. Click here to view full image |
SOD: superoxide dismutase, GSH: glutathione, CAT: catalase, DXN: doxorubicin, VVE: Vitis vinifera extract, ns: non significant.
Values were expressed as mean ± SEM, n=6. Data were analyzed by one way ANOVA followed by Dunnett’s multiple comparison test.
***P < 0.001, **P < 0.01, *P < 0.05 & ns vs. DXN group.
^P < 0.05 vs. normal control group.
Free radicals ROS (reactive oxygen species) and RNS (reactive nitrogen species) are generated by our body by various endogenous systems upon exposure to different physiochemical conditions, or pathological states. A balance between free radicals and antioxidants is necessary for proper physiological function. If the free radicals overwhelm the body’s ability to regulate them, a condition known as oxidative stress ensues. Free radicals thus adversely alter lipids, proteins, and DNA and trigger a number of human diseases. Hence, application of an external source of antioxidants can assist in coping this oxidative stress (Lobo et al., 2011).
Among all the therapeutic modalities adopted to attenuate DXN toxicity provide the most promising results from combining the drug with a myriad of antioxidants in an attempt to abate oxidative damage in heart, liver and kidney tissues and hence to abrogate the tissue injury.
The present study was to evaluate the potential protective effect of the hydroalcoholic extract of Vitis vinifera against DXN-induced oxidative stress in heart, liver and kidney tissues. It is evident from the results of the present investigation that supplementation of Vitis vinifera hydroalcoholic extract protected the animals against the toxic effects of DXN.
 |
Figure 3 (A). Effect of hydroalcoholic extract of Vitis vinifera on hepatic GSH, SOD, MDA and catalase. Click here to view full image |
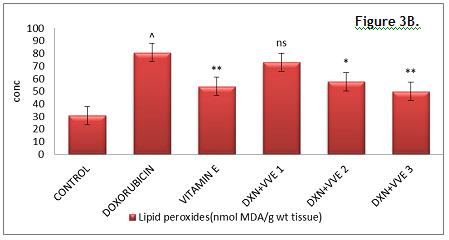 |
Figure 3 (B). Effect of hydroalcoholic extract of Vitis vinifera on hepatic GSH, SOD, MDA and catalase. Click here to view full image |
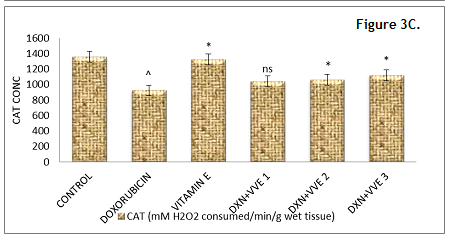 |
Figure 3 (C). Effect of hydroalcoholic extract of Vitis vinifera on hepatic GSH, SOD, MDA and catalase. Click here to view full image |
GSH: glutathione, SOD: superoxide dismutase, DXN: doxorubicin, VVE: Vitis vinifera extract, CAT: catalase, ns: non significant.
Values were expressed as mean ± SEM, n=6. Data were analyzed by one way ANOVA followed by Dunnett’s multiple comparison test.
***P < 0.001, **P < 0.01, *P < 0.05 & ns vs. DXN group.
^P < 0.05 vs. normal control group.
Vitis vinifera are an important dietary antioxidant; it significantly decreases the adverse effect of reactive species such as ROS & RNS that can cause oxidative damage to macromolecules such as lipids, DNA and proteins. Vitis vinifera possess potent antioxidant capacity, capable of scavenging/neutralizing an array of reactive oxygen species hydroxyl, alkoxyl, peroxyl, superoxide anion, hydroperoxyl radicals and reactive nitrogen radicals such as nitrogen dioxide, nitroxide, peroxynitrite at very low concentration (En-Qin Xia et al., 2010). DXN-induced toxicity includes one electron reduction of DXN lead to the formation of corresponding semi-quinone free radicals in heart, liver and kidney tissues by CYP-450 and flavin monoxigenase. In the presence of oxygen, these free radicals rapidly donate their electron to oxygen or react with molecular oxygen and initiate cascade of reaction producing ROS. Free radical generation and lipid peroxidation have been suggested to be responsible for DXN-induced toxicity (Chularojmontri et al., 2005; Xu et al., 2001).
Moreover, heart tissue especially is susceptible to the free radical injury detoxifying enzymes such as SOD, catalase, and GSH and less oxygen reserve. Further, DXN also has a high affinity for the phospholipids component of mitochondrial membrane in cardiac myocytes, leading to accumulation of DXN in heart tissue.
The cellular GSH level is closely related to lipid peroxidation and disturbances of Ca++ influx induced by toxic agents. DXN administration induced oxidative stress in cardiac tissue as manifested by the alteration observed in the cardiac antioxidant defense system both enzymatic and nonenzymatic. The modulation of antioxidant enzyme activities followed by DXN administration has been discussed in many studies. The association between elevated cardiac content of MDA and lowered cardiac content of GSH found in the study strongly proves the oxidative damage caused by DXN administration. It is well documented that long-term treatment by DXN causes irreversible, severe, and potentially life-threatening cardiac damage. The mechanism involved in such toxicity has been documented by many investigators. The involvement of oxygen free radicals oxidative stress has been strongly accepted as crucial factors in the pathogenesis of DXN-induced cardiac damage (Hrdina et al., 2000). Alanine transaminase and aspartate transaminase are regarded as markers of liver injury, since liver is the major site of metabolism. Several researchers have reported decreased activities of alanine transaminase and aspartate transaminase in liver due to DXN toxicity (Padmavathi et al., 2007), which is consistent with present findings. Decline in the activities of liver alkaline phosphatase in DXN injected animals’ noticeably demonstrated cellular damage which correlates with the present findings. Elevation of serum levels of alanine transaminase and aspartate transaminase in DXN injected animals is attributed by lipid peroxidation in the liver. Influence of DXN toxicity, therefore, reveals leakage of these enzymes from damaged liver cells.
The results of the renal function test revealed that DXN administration produced intrinsic acute renal failure, which was evident from the elevated levels of serum urea and creatinine. Anticancer therapy usually demolishes the physiological homoeostasis and affects multiple organs during treatment process. Effective anticancer therapy with anthracyclines is limited because of its toxicity to various organs including kidneys (Hertzan et al., 2000). The toxicity has been attributed to radical formation and oxidant injury. Nephrotoxic action of DXN is also considered to be via drug-induced free radical generation (Shah et al., 1989). The formation of free radicals as well as an increase in response to DXN treatment has already been documented. The disturbance in oxidant-antioxidant systems results in tissue injury that is demonstrated with protein oxidation in tissue and protein oxidation in renal tissue is recognized as one of the possible biochemical mechanisms of DXN-induced nephrotoxicity, and we have found that DXN treatment raised Po (protein oxidation product) in rat kidney.
DXN administration alters the levels of various biochemical parameters like increase of LDH, Troponin- I, SGPT, SGOT, ALP, BUN, serum creatinine, decrease of total protein levels and also alters the antioxidant levels like decrease of SOD, GSH, catalase and increase in the lipid peroxidation. These alterations lead to cardiotoxicity, hepatotoxicity and nephrotoxicity. But the pretreatment of hydroalcoholic extract of Vitis vinifera extract significantly reduced levels of various biochemical parameters like LDH, Troponin- I, SGPT, SGOT, ALP, BUN, Serum creatinine and lipid peroxidation, and significantly increased total protein levels and antioxidants levels like SOD, GSH and catalase as that of the standard Vitamin E. These results could confirm that the hydroalcoholic extract of Vitis vinifera is having the protective effect against the cardiotoxicity, hepatotoxicity and nephrotoxicity induced by the DXN. The higher amounts of the polyphenolics and flavonoids, resveratrol compounds which are present in the extract are responsible for the free radical scavenging activity.
Most of the past antioxidant studies of Vitis vinifera are confined to the either leaves or seed or skin but not whole fruit. Whole fruit shows higher phenolic contents and antioxidant activity than seeds and leaves. This study has shown that Vitis vinifera hydroalcoholic extract from the fruits had significant antioxidant activity and caused a significant reversal of the DXN-induced changes in the oxidative biomarkers in serum and tissues. Observed changes could be due to the different polyphenols, flavonoids, and flavones present in the extract. The obtained result proves that the whole fruit extract of Vitis vinifera has free radical scavenging and antioxidant properties.
 |
Figure 4 (A & B).Effect of hydroalcoholic extract of Vitis vinifera on renal GSH, catalase and SOD. Click here to view full image |
SOD: superoxide dismutase, GSH: glutathione, CAT: catalase, DXN: doxorubicin, VVE: Vitis vinifera extract, ns: non significant.
Values were expressed as mean ± SEM, n=6. Data were analyzed by one way ANOVA followed by Dunnett’s multiple comparison test.
***P < 0.001, *P < 0.05 & ns vs. DXN group. ^P < 0.05 vs. normal control group.
CONCLUSION
The protecting effect of hydroalcoholic extract of Vitis vinifera is due to free radical scavenging and iron-chelating properties, hydrogen-donating radicals, scavenger by the scavenging lipid alkoxyl and peroxyl radical. On the basis of these findings, it may be worthy to suggest the subsequent consumption of Vitis vinifera (black grape) prior to the DXN will prevent from harm to the heart, liver, and kidney in cancer chemotherapy. Further studies are required to isolate the potential phytoconstituents present in the extract.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGEMENTS
he authors are thankful to the authorities of Malla Reddy College of Pharmacy, Secunderabad, AP, India for providing support to the present study.
REFERENCES
Al-majed A, Gado A, AL-shbanah O, Mansour MA. Aplha lipoic acid ameliorates myocardial toxicity induced by doxorubicin. Pharmacol Res. 46, 499–503, 2002.
Bagachi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamin C and E, and a grape seed proanthocyanidins extract in vitro. Res Common Mol Pathol Pharmacol. 95, 179, 1997.
Billingham ME, Mason JW, Bristow MR, Daniels JR. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat Rep. 62, 865-872, 1978.
Blum RH, Carter SK. Adriamycin. A new anticancer drug with significant clinical activity. Ann Intern Medicine. 80, 249-259, 1974.
Bowers LD. Kinetic serum creatinine assays I. The role of various factors in determining specificity. Clin Chem. 26, 551-554, 1980.
Brown JC, Huang G, Haley-Zitlin V, Jiang X. Antibacterial effects of grape extracts on Helicobacter pylori. Appl Environ Microbiol. 75, 848-852, 2009.
Chularojmontri L, Wattanapitayakul SK, Herunsalee A, Charuchongkolwongse S, Niumsakul S, Srichairat S. Antioxidative and cardioprotective effects of Phyllanthus urinaria L on doxorubicin-induced cardiotoxicity. Biol Pharm Bull. 28, 1165–1171, 2005.
Dragsted LO. Natural antioxidants in chemoprevention. Arch Toxicol Suppl. 20, 209, 1998.
En-Qin Xia. Gui-Fang Deng, Ya-Jun Guo, Hua-Bin Li. Biological activities of polyphenols from grapes. Int J Mol Sci. 11, 622-646, 2010.
Fiske CH, Subbarow Y. The colorimetric determination of phosphatase. J Biol Chem. 66, 375–400, 1925.
Gillick J, Giles S, Bannigan J, Puri P. Cell death in the early adriamycin rat model. Pediatr Surg Int. 18, 576-580, 2002.
Greenspan P, Bauer JD, Pollock SH, Gangemi JD, Mayer EP, Ghaffar A, Hargrove JL, Hartle DK. Antiinflammatory properties of the muscadine grape (Vitis rotundifolia). J Agric Food Chem. 253, 8481-8484, 2005.
Henry RJ, Chiamori N, Goiub OJ, Berkman S. Revised spectrophotometric methods for the determination of glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase and lactic acid dehydrogenase. Am J Clin Pathol. 1960, 34, 381–98, 1960.
Hertzan-Levy S, Fish R, Skutelsky E Wollman Y, Chernichovsky T, Polak-Charcon S, Schwartz D, Blum M, Cabili S, Iaina A. Glomerular basement membrane anionic sites in Adriamycin nephropathy: effect of saline loading and nitric oxide modulation. Nephron. 84, 354–361, 2000.
Hrdina R, Gersl V, Klimtova I, Simunek T, Mach J, Adamcova M. Anthracycline-induced cardiotoxicity. Acta Medica. 43, 75–82, 2000.
Kim TJ, Silva JL, Weng WL, Chen WW, Corbitt M, Jung YS, Chen YS. Inactivation of Enterobacter sakazakii by water-soluble muscadine seed extracts. Int J Food Microbiol. 28, 295-299, 2009.
Koima S, Icho T, Hayashi M, Kajiwara Y, Kitabatake K, Kubota K. Inhibitory effect of 5, 6, 7, 8-tetrahydroneoterin on adriamycin-induced cardiotoxicity. J Pharmacol Exp Ther. 266, 1699-1704, 1993.
Krishenbaum LA, Singal PK. Increase in endogenous antioxidant enzymes protects hearts against reperfusion injury. Am J Physiol. 265, H484-H493, 1993.
Liu LL, Li QX, Xia L, Li J, Shao L. Differential effects of dihydropyridine calcium antagonists on doxorubicin-induced nephrotoxicity in rats, Toxicology, 231, 81–90, 2007.
Lobo V, Patil A, Phatak A. Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 118, 26, 2011.
Melanson SE, Tanasijevic MJ, Jarolim P. Cardiac troponin assays: a view from the clinical chemistry laboratory. Circulation. 116, e501–504, 2007.
Mertens-Talcott SU, Zadezensky I, De Castro WV, Derendorf H, Butterweck V. Grapefruit-drug interactions: can interactions with drugs be avoided? J Clin Pharmacol. 46, 1390-1416, 2006.
Nagi MN, Mansour MA. Protective effects of thymoquinone against doxorubicin induced cardiotoxicity in rat a possible mechanism of protection. Pharmacol Res. 41, 283–289, 2000.
Shinde N, Jagtap A, Undale V, Kakade S, Kotwal S, Patil R. Protective effect of Lepidium sativum against Doxorubicin-induced nephrotoxicity in rats. Res J Pharm Biol Chem Sci. 1, 42-49, 2010.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95, 351–358, 1979.
Poudel PR, Tamura H, Kataoka I, Mochioka R. Phenolic compounds and antioxidant activities of skins and seeds of five wild grapes and two hybrids native to Japan. J Food Comp Anal. 21, 622-625, 2008.
Reitman S, Frankel SA. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 28, 56–63, 1957.
Saber A, Sakr Hoda A, Mahran Hawaze, Lamfon, A. protective effect of ginger (Zingiber officinale) on adriamycin-induced hepatotoxicity in albino rats. J Medicin Plants Res. 5, 133-140, 2011.
Shah SV. Role of reactive oxygen metabolites in experimental glomerular disease. Kidney Int. 35, 1093–1106, 1989.
Shiromwar SS, Chidrawar VR. Combined effects of p-coumaric acid and naringenin against doxorubicin-induced cardiotoxicity in rats. Pharmacog Res 3, 214-219, 2011.
Uchiyama M, Mihara M. Determination of malonaldialdehyde precursor in tissue by thiobarbituric acid test. Anal Biochem. 86, 271–278, 1978.
Wapstra FH,Van Goor HP, De Jong, Navis G, De Zeeuw D. Dose of doxorubicin determines severity of renal damage and responsiveness to ACE-inhibition in experimental nephrosis. J Pharmacol Toxicol Methods. 41, 69–73, 1999.
Xu MF, Tang, PL, Qian ZM, Ashraf M. Effects by doxorubicin on the myocardium is mediated by oxygen free radicals. Life Sci. 68, 889–901, 2001.
Yilmaz S, Atessahin A, Sahna E, Karahan I, Ozer S. Protective effect of lycopene on adriamycin- induced cardiotoxicity and nephrotoxicity. Toxicology. 218, 164-171, 2006.









 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.