Srinivasa Rao A1, Mohammed Fazil Ahmed2*
1Bhaskar Pharmacy College, Yeknapally, Moinabad (Mandal), RR (Dist), Hyderabad- 500075, India.
2Nizam Institute of Pharmacy & Research Center, Deshmukhi, Pochampally (M), Near Ramoji Film City, Nalgonda-508284, AP, India.
ORIGINAL RESEARCH ARTICLE
ARTICLE INFORMATION
Article history
Received: 05 December 2013
Revised: 15 December 2013
Accepted: 20 December 2013
Early view: 29 December 2013
*Author for correspondence
E-mail: [email protected]
Mobile/ Tel.: 0000000000
Keywords:
Periwinkle
Silymarin
Vinca rosea
SGOT
SGPT
ALP.
Background: The main aim of the present study is to evaluate the hepatoprotective activity of ethanolic extract of Catharanthus rosea L. leaves against simvastatin-induced hepatotoxicity in rats.
Materials and methods: The phytochemical study was also carried out on the ethanolic extract of Catharanthus rosea leaves. Hepatotoxicity was induced by simvastatin (20 mg/kg p.o. for 30 days) in Wistar rats. The hepatoprotective effect was evaluated by extract of Catharanthus rosea leaves (300 mg/kg and 500 mg/kg for 30 days).
Results: : The observation of the phytochemical investigations includes the presence of alkaloids, tannins, saponins, glycosides, phenols, and flavonoids. There was significant changes in biochemical parameters e.g. increases in serum glutamate pyruvate transaminase (SGPT), Serum glutamate oxaloacetate transaminase (SGOT), alanine phosphatase (ALP), serum bilirubin and decrease the total protein contents in Catharanthus rosea (300 mg/kg and 500 mg/kg) treated animals. The results were comparable to standard hepatoprotective drug Silymarin (25 mg/kg).
Conclusion: Thus the present study ascertains that the ethanolic extract of Catharanthus rosea leaves possesses significant hepatoprotective activity.
INTRODUCTION
The liver is the key organ regulating homeostasis in the body. It is involved with almost all the biochemical pathways related to growth, fight against diseases, nutrient supply, energy provision and reproduction (Ward et al., 1999). Within the human body, millions of chemical reactions are occurring constantly which require oxygen. Reactive oxygen spices (ROS), sometimes called active oxygen species, are various forms of activated oxygen, which include free radicals such as superoxide ions (O2-) and hydroxyl radicals (OH-), as well as non-free-radicals species such as hydrogen peroxide (H2O2) (Halliwell et al., 1995; Squadriato et al., 1998). They tend to attack the healthy cells, DNA as well as proteins and fats, causing them to deteriorate. Antioxidants are compounds that protect cells against the damaging effects of reactive oxygen specious, such as singlet oxygen, super oxide, peroxyl radicals, hydroxyl radicals and peroxynitrite. An imbalance between antioxidants and reactive oxygen species results in oxidative stress, leading to cellular damage. Oxidative stress has linked to cancer, ageing, atherosclerosis, and ischemia injury, inflammation and neurodegenerative diseases like parkinsonism and alzheimer (Donald et al., 1987).
More than 900 drugs have been implicated in causing liver injury and it is the most common reason for a drug to be withdrawn from the market (Friedman et al., 2003). Drug-induced liver injury is responsible for 5% of all hospital admissions and 50% of all acute liver failures (Friedman et al., 2003; Ostapowicz et al., 2002). Simvastatin causes oxidative stress mediated hepatotoxicity by depleting antioxidant enzymes (Vaghasiya et al., 2008). Simvastatin hepatotoxicity is hypothesized to occur due to drug-drug interactions (Ricaurte et al., 2006; Kanathur et al., 2001). Simvastatin (lipid lowering agent) competitively inhibits HMG-Co A (3-hydroxy-3 methylglutaryl coenzyme A) to mevalonate. Mevalonate is also a precursor of Coenzyme Q10 (CoQ10). Thus, treatment with statins could also lower its levels. CoQ10 acts as an antioxidant, has membrane stabilizing effects, and is important for cellular mitochondrial respiration, which is essential for energy production in organs (Frei et al., 1990; Stocker et al., 1991).
Catharanthus rosea, which is commonly known as ‘periwinkle’ or ‘Vinca rosea’ belongs to family Apocynaceae and is an important source of indole alkaloids, which are present in all plant parts. The physiologically important and antineoplastic alkaloids namely Vincristine and Vinblastine are mainly present in the leaves whereas antihypertensive alkaloids such as ajmalicine, serpentine, and reserpine are reported to be present in the roots (Mishra et al., 2001). Vincristine and Vinblastine alkaloids are used in the treatment of various types of lymphoma and leukemia (Farnsworth et al., 1968; Svoboda et al., 1975). Crude decoction of (200 mg and 1 g herb/ml water) Catharanthus showed a moderate anti-angiogenesis effect in vitro (Wang et al 2004). Numerous animal studies have shown that ethanolic extracts of leaves and flowers of Catharanthus lower blood glucose levels (Ghosh and Gupta, 1980; Chattopadhyay et al., 1991, 1992). The main objective of this study was to assess the hepatoprotective effect of Catharanthus rosea in simvastatin-induced hepatotoxicity.
MATERIAL AND METHODS
Plant collection and identification
The plant material Catharanthus rosea or Vinca rosea L. obtained from Mount Opera Garden, Near Ramoji Film City, Nalgonda Dist., AP, India. The plant was identified and authenticated by Research Office (Botanist), Department of Botany, Anwar-ul-uloom College of Pharmacy, Hyderabad, India.
Extraction
The leaves were dried under shade and powdered in a mechanical grinder. The powdered material (250 gms) was extracted successively in ethanol using Soxhlet apparatus at 55 °C for 18 h. The extracts was concentrated in vacuo and kept in a vacuum dessicator for complete removal of solvent and weighed.
Phytochemical investigation
The phytochemical studies of Catharanthus rosea or Vinca rosea L. leaves were performed for testing the different chemical groups such as alkaloids, tannins, glycosides and saponins etc present in extracts (Trease et al., 1978; Kokate et al., 1990; Khandelwal et al., 2006).
Experimental Animals
Wistar albino rats (150-200 g) of both sexes were obtained from the animal house of Nizam Institute of Pharmacy, Deshmukhi, Ramoji film city, Hyderabad, India. Before and during the experiment, rats were fed with standard diet (Gold Moher, Lipton India Ltd). After randomization into various groups and before initiation of experiment, the rats were acclimatized for a period of 7 days under standard environmental conditions of temperature, relative humidity, and dark/light cycle. Animals described as fasting were deprived of food and water for 16 h ad libitum. All animal experiments were carried out in accordance with the guidelines of CPCSEA and study was approved by the IAEC (Institutional Animal Ethical Committee; 1330/ac/10/CPCSEA).
Experimental design for hepatoprotective activity
Animals were divided into five groups, each comprising 6 rats.
Group I : Normal control (saline, p.o.)
Group II : Simvastatin (20 mg/kg, p.o.)
Group III: Simvastatin (20 mg/kg, p.o.) + Catharanthus rosea extract (300 mg/kg, p.o.)
Group IV: Simvastatin (20 mg/kg, p.o.) + Catharanthus rosea extract (500 mg/kg, p.o.)
Group V : Simvastatin (20 mg/kg p.o.) + Silymarin
(25 mg/kg, p.o.)
Animals were divided into five different groups, each having 6 rats and treated accordingly. Group I rats fed with a normal standard diet for 30 days. Group II rats received Simvastatin (SMT) (20 mg/kg, p.o. alone for 30 days). Group III and IV rats received SMT along with Catharanthus rosea extracts (300 mg/kg and 500 mg/kg, p.o. respectively for 30 days) and Group V rats received SMT along with silymarin (20 mg/kg/p.o. for 30 days) (Vaghasiya et al., 2009). On the 31st day, all the animals were sacrificed by mild ether anesthesia.
Blood biochemistry
Blood samples were collected in glass tube from retro-orbital puncture to obtain haemolysis free clear serum for the analysis of SGOT and SGPT (Reitman et al., 1957), ALP (Walter et al., 1974.) and bilirubin (Malloy et al., 1937) and other biochemical investigations. Serum total protein was measured according to the method of Lowry et al., 1951.
Histopathology
Histopathology of liver was carried out by a modified Luna method (Luna, 1999). In brief, the autopsied livers were washed in normal saline and fixed in 10% formalin for 2 days followed with bovine solution for 6 h. Then the livers were paraffin embedded and 5 μ thickness microtone sections were made (Krajian 1963). The sections were processed in alcohol-xylene series and stained with haematoxylin and eosin. The slides were studied under a light micro-scope for any histological damage/protection.
Statistical analysis
The data are represented as mean + S.E.M. Students’ ‘t’-test is used for statistical analysis of blood serum parameters and for statistical analysis of liver enzymes.
RESULTS
The phytochemical evaluation of Catharanthus rosea shows the presence of tannins, saponins, glycosides, phenols, flavonoids and while steroids and terpenes were absent in Catharanthus rosea (Table 1).
The effect of ethanolic extract of Catharanthus rosea leaves on serum transaminases, alkaline phosphates, bilirubin and total protein level in simvastatin intoxicated rats are summarized in Table 2. There was a significant increase in bilirubin levels, SGOT, SGPT and ALP, in simvastatin intoxicated group compared to the normal control group. The total protein levels were significantly decreased in simvastatin intoxicated rats.
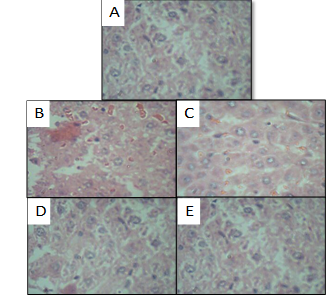 |
Figure 1.(A) Section of liver of (Normal control) (B) Section of liver of (Negative control; Simvastatin); (C) Section of the liver of Test-low dose; Catharanthus rosea + Simvastatin; (D) Test-high dose; Catharanthus rosea + Simvastatin); (E) Section of liver of Positive control (Simvastatin + Silymarin). Click here to view full image |
 |
Table 1 Phytochemical analysis of ethanolic extracts of Catharanthus rosea leaves. Click here to view full image |
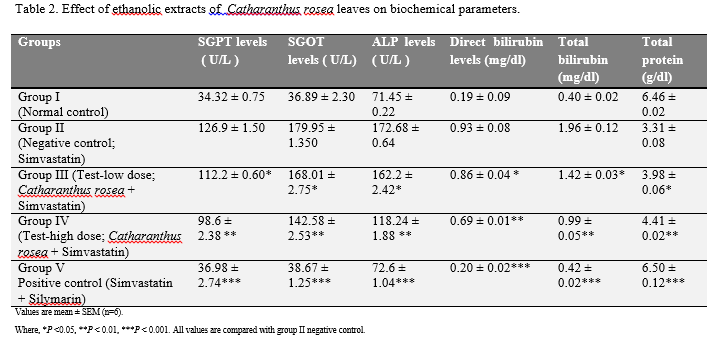 |
Table 2 Effect of ethanolic extracts of Catharanthus rosea leaves on biochemical parameters. Click here to view full image |
On the other hand the groups which received both Catharanthus rosea extract (300 mg/kg, and 500 mg/kg,) + simvastatin (20 mg/kg, p.o.) (Group III & IV) and simvastatin (20 mg/kg, p.o.) + silymarin (25 mg/kg, p.o.) (Group V) showed significantly decreased the elevated serum marker enzymes when given orally and reversed the altered total protein to almost normal level [Table 2 and Fig 1(A-E)].
DISCUSSION
The liver can be injured by many chemicals and drugs (Leo et al., 1982). During hepatic damage, cellular enzyme like SGOT, SGPT, ALP and serum bilirubin present in the liver cell, leak into the serum resulting to increase in concentration (Deb, 1998). The protective effect of the component of PHF has also been observed in several experimental studies (Sandhir et al., 1999; Mathur et al., 1994). In the previous studies, it was reported that simvastatin caused oxidative stress mediated hepatotoxicity (Vaghasiya et al., 2008). The protection of liver cells against toxic materials including drugs, lipid peroxidation, and free radical injury may decrease inflammation (Yang et al., 2000). It is reported that phenols are responsible for the variation in the antioxidant activity of the plant (Cai et al., 2004). They exhibit antioxidant activity by inactivating lipid free radicals or preventing decomposition of hydroperoxides into free radicals (Pitchaon et al., 2007; Pokorny et al., 2001). Phenolic compounds are considered to be the most important antioxidative components of herbs and other plant materials, and a good correlation between the concentrations of plant phenolic and the total antioxidant capacities has been reported (Madsen et al., 1998; Pellegrini et al., 2000).
In simvastatin group histological changes such as steatosis (fatty changes in hepatocytes) and perivenular fibrosis were observed. While in ethanolic extracts of Catharanthus rosea (300 mg/kg and 500mg/kg, p.o) prevented these histological changes, further indicating their hepatoprotective activity.
CONCLUSION
The results of present study demonstrate that Catharanthus rosea leaves extract has potent hepatoprotective activity against simvastatin induced liver damage in rats. The results also imply that the hepatoprotective effects of Catharanthus rosea may be due to its antioxidant property. Further investigation is in progress to determine the exact phytoconstituents responsible for hepataprotective effect.
CONFLICT OF INTEREST
None declared.
REFERENCES
Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 74, 2157-2184, 2004.
Chattopadhyay RR, Sarkar SK, Ganguli S. Hypoglycemic and antihyperglycemic effect of leaves of Vinca rosea Linn. Indian J Physiol Pharmacol. 35, 145-51, 1991.
Chattopadhyay RR, Sarkar SK, Ganguli S. Antiinflammatory and acute toxicity studies with leaves of Vinca rosea Linn in experimental animals. Indian J Physiol Pharmacol. 36, 291–2, 1992.
Deb AC. Fundamental of Biochemistry. 7th edition. New Central Book Agency: Kolkata, India, 1998.
Donald RB, Cristobal M. Antioxidant activities of flavonoids. PhD thesis submitted to Department of Environmental and Molecular Toxicology, Oregon State University: Oregon, USA, 1987.
Farnsworth NR, Svoboda GH, Blomster RN. Antiviral activity of selected Catharanthus alkaloids, J Pharm Sci. 57, 2174-2175, 1968.
Frei B, Kim M, Ames B. Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations. Proc Natl Acad Sci. 87, 4879-4883, 1990.
Friedman SE, Grendell JH, McQuaid KR. Current Diagnosis & Treatment in Gastroenterology. Lang Medical Books, McGraw-Hill: New York, pp 664–679, 2003.
Ghosh RK, Gupta I. Effect of Vinca rosea and Ficus racemososuson in hyperglycemia in rats. Indian J Animal Health. 19, 145–148, 1980.
Halliwell B. How to characterize anantioxidant: an update. Biochem Soc Symp. 61, 73-101, 1995.
Kanathur N, Mathai MG, Byrd RP Jr, Fields CL, Roy TM. Simvastatin-diltiazem drug interaction resulting in rhabdomyolysis and hepatitis. Tennessee Med. 94, 339–341, 2001.
Khandelwal, KR. Practical Pharmacognosy Techniques and Experiments, 16th edition. Nirali Prakashan: Pune, India, 149-156, 2006.
Kokate CK, Purohith AP, Gokhale SB. Pharmacognosy, Nirali Prakashan: Pune, India, 120, 1990.
Leo MA, Arai M. Hepatotoxicity of vitamin A and CCl4. Gastroenterology. 82, 194-205, 1982.
Lowry OH, Rosebrough NJ, Farr AL. Protein measurement with the folin-phenol reagent. J Biol Chem. 193, 265-275, 1951.
Madsen HL, Nielsen BR, Bertelsen G, Skibsted LH. Screening of antioxidative activity of spices. Food Chem. 57, 331-337, 1996.
Malloy HT, Evelyn KA. The determination of bilirubin with the photoelectric colorimeter. J Biol Chem. 119, 481-490, 1937.
Mathur S. Role of Liv-52 in protection against beryllium intoxication. Biol Trace Elem Res. 41, 201-215, 1994.
Mishra P, Uniyal GC, Sharma S. Pattern of diversity for morphological and alkaloid yield related trades among the periwinkle Catharanthus roseus accessions collected from in and around Indian Subcontinent. Genetic Res Crop Evol. 48, 273-286, 2001.
Ostapowicz G, Fontana RJ, Schiødt FV. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 137, 947–954, 2002.
Pellegrini N, Simonetti P, Gardana C, Brenna O. Brighenti activity of Vini novelli (Young red wines). J Agricul Food Chem. 48, 732-735, 2000.
Pitchaon M, Suttajit M, Pongsawatmani R. Assessment of phenolic content and free radical scavenging capacity of some Thai indigenous plants. Food Chem. 100, 1409-1418, 2007.
Pokorny J, Yanishlieva N, Gordon M. Antioxidants in Food, Practical Applications. Woodhead Publishing Limited: Cambridge, pp 1-3, 2001.
Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 28, 56-63, 1957.
Ricaurte B, Guirguis A, Taylor HC, Zabriskie D. Simvastatin-amiodarone interaction resulting in rhabdomyolysis, azotemia, and possible hepatotoxicity. Annals of Pharmacother. 40, 753–757, 2006.
Sandhir R, Gill KD. Hepatoprotective effects of Liv-52 on ethanol-induced liver damage in rats. Indian J Exp Biol. 37, 762-766, 1999.
Squadriato GL, Pelor WA. Oxidative chemistry of nitric oxide: The roles of superoxide, peroxynitrite, andcarbon dioxide. Biol Med. 25, 392-403, 1998.
Stocker R, Bowry VW, Frei B. Ubiquinone-10 protects low density lipoprotein more efficiently against lipid peroxidation than does alphatocopherol. Proc Natl Acad Sci. 88, 1646-1650, 1991.
Svoboda GH, Blake DA, The phyto-chemistry and pharmacology of Catharanthus roseus (L.) G Don Inc. In: Taylor, WJ, Farnsworth, NR (eds.): The Catharanthus alkaloids. Marcel Decker: New York, pp 45-84, 1975.
Trease GE, Evans WC. A Text book of Pharmacognosy, 11th edition. Bailliere Tidall: London, pp 530, 1978.
Vaghasiya J, Rathod S, Bhalodia Y, Manek R, Malaviya S, Jivani N. Protective effect of polyherbal formulation on simvastatin hepatotoxicity in rats. J Young Pharm. 1, 57-62, 2009.
Vaghasiya JD, Bhalodia YS, Manek RA, Gohil TA, Rathod SP. Oxidative stress mediated hepatotoxicity produced by simvastatin. Pharmacology online 3, 495-503, 2008.
Walter K, Schutt C. Acid and alkaline phoshatases in serum. In: Verlag Chemic Weinheim, In: Hans Ulrich Bergmeyer (eds.). Method Enzymatic Anal. Academic Press Inc: New York, pp 856-864, 1974.
Wang S, Zheng Z, Weng Y. Angiogenesis and anti-angiogenesis activity of Chinese medicinal herbal extracts. Life Sci. 74, 2467-2478, 2004.
Yang H, Chen Y, Xu R, Shen W, Chen G. Clinical observation on the long-term therapeutic effects of traditional Chinese medicine for treatment of liver fibrosis. J Tradit China Med. 20, 247-2450, 2000.









 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.