Mohammad Mansoor, Chitta Sai Brahmini*, Srinivasa Rao D.
K.C. Reddy Institute of Pharmaceutical Sciences, Guntur, Andhra Pradesh-522 348, India.
ORIGINAL RESEARCH ARTICLE
Volume 3, Issue 2, Page 98-101, May-August 2015.
Article history
Received: 20 July 2015
Revised: 13 August 2015
Accepted: 14 August 2015
Early view: 19 August 2015
*Author for correspondence
E-mail: [email protected]
Background: In traditional medicine, different parts of Ginkgo biloba Linn are highly valued in the treatment of human diseases including antimicrobial, diuretic, anti-inflammatory activity, hepatoprotective etc. In the present study nephroprotective activity of ethanolic leaves extract of Ginkgo biloba were studied, against gentamicin induced nephrotoxicity in rats.
Material and methods: Gentamicin induced kidney damage, showed significant increase in renal parameters like serum creatinine, serum urea, serum uric acid, blood urea nitrogen and weight of kidney.
Results: Administration of ethanolic leaves extracts of Ginkgo biloba 200 mg/kg, p.o. and 400 mg/kg, p.o. sufficiently decreased altered parameters near to normal level indicating the nephroprotective activity of Ginkgo biloba.
Conclusion: Daily treatment with Ginkgo biloba (200 mg/kg and 400 mg/kg) for 8 days conferred nephroprotection on gentamicin induced rats in a dose dependent fashion offered maximum protection.
Keywords: Ginkgo biloba, Nephrotoxicity, Gentamicin, Ethanol, Nephroprotectivity.
INTRODUCTION
Renal failure is one of the most a common clinical syndrome. Kidney failure is defined as, a rapid decline/decrease in kidney function, which resulting in abnormal retention of blood urea and serum creatinine, which must be excreted. Renal disease is the ninth leading cause of death. Approximately, 19 million adults have chronic renal disease and an estimated 80,000 persons have chronic kidney failure diagnosed annually in India. Recent literature showed a prevalence of chronic renal failure of 0.16% and 0.79% in India. Renal replacement is the only therapy in the end stage of renal failure. In case of nonavailability of kidney, the only alternative is dialysis. No exclusive drug has been reported so far, as such in any category of medical treatment. Nephrotoxicity is the third most common problem of the renal system with an estimated lifetime risk of 2-5% in Asia, 8-15% in Europe and America and around 20% in the Middle East (Priyadarsini et al., 2012).
Ginkgo biloba L., due to its survival over millions of years, it is considered as living fossil (Braquet, 1988; Boonkaew and Camper 2005). One of the greatest properties of Ginkgo is that it is resistance to serious plant diseases. Ginkgo contains a number of biologically active compounds for its defence against insects, bacteria, and fungi (Bombardelli et al., 2000). G. Biloba leaves extracts used in the treatment of cerebral insufficiency, was reported in the 1960s and designated EGb 761 (Maurer et al., 1997). This preparation is in the ratio of acetone:water (60 : 40) extract from the dried leaves(Drieu 1986). A number of secondary metabolites, like organic acids, terpenoids, polyphenols, and amino acids have been isolated from this plant. However, the main bioactive constituents of Ginkgo biloba are trilactones, terpene and flavonoid glycosides which are considered responsible for the pharmacological activities (Singh et al., 2008). Ginkgo biloba leaves have been used as agents for improving cerebral circulation and they possess antiparasitic, antitumor and antiviral activities (Atzori et al., 1993; Oken et al., 1998, Wadsworth and Koop 2001). The role of the Ginkgo biloba extract used in the treatment of diseases involving in free radicals and oxidative damage has also been suggested (Bridi et al., 2001, Pitchumoni Doraiswamy, 1998).
MATERIALS AND METHODS
Preparation of plant extract
100 g of Ginkgo biloba leaves were powdered, dried and continuously extracted using Soxhlet apparatus for 48hrs with ethanol as a solvent. The collected crude extract Ginkgo biloba was stored at 0-4 °C until used.
Preliminary phytochemical screening
Preliminary phytochemical investigation of Ginkgo biloba leaves was carried out on for detection of various phytochemicals by following standard methods described in practical Pharmacognosy by RK Khandelwal and CK Kokate.
Experimental animals
Wistar albino rats (150-200 g) of either sex were obtained from the animal house. During and before the experiment, rats were fed with standard diet (Gold Moher, Lipton India Ltd.). After randomization, the rats into various groups and before initiation of experiment, the rats were acclimatized for 7 days under standard environmental conditions of temperature, and dark/light cycle and relative humidity. Rats described as fasting, when deprived of food and water for 16 h ad libitum. All experiments on rats were carried out in accordance with the guidelines of CPCSEA and study ware approved by the IAEC (Institutional animal ethical committee).
Gentamicin induced nephrotoxicity in rats (Lakshmi et al., 2009)
The albino rats (150-200 g) of both sexes will be randomly divided into 5 groups of 6 each. The different groups as described below.
Group I: Vehicle control; Group II: Nephrotoxicity group (Gentamicin 100 mg/kg); Group III: Ginkgo biloba leaves extract (200 mg/kg, p.o.)+Gentamicin (100 mg/kg, i.p.); Group IV: Ginkgo biloba leaves extract (400 mg/kg, p.o.)+Gentamicin (100 mg/kg, i.p.); Group V: Standard polyherbal drug cystone (5 ml/kg; p.o.)+Gentamicin (100 mg/kg, i.p.).
Experimental procedure
The gentamicin treated groups received 100 mg/kg/day gentamicin by the intraperitoneal (i.p.) route. Rats in the control group I were given sterile saline solution for 8 days. Group II received 100 mg/kg gentamicin i.p. alone for 8 days. Group III received 100 mg/kg gentamicin i.p. and Ginkgo biloba 200 mg/kg/ p.o. for eight days and Group IV received 100 mg/kg/ gentamicin i.p. and Ginkgo biloba 400 mg/kg/p.o. for eight days. Group V received 100 mg/kg/ gentamicin i.p. and standard polyherbal drug cystone (5 ml/kg; p.o.) for eight days.
After dosing on the 8th day, blood samples were collected via cardiac puncture method at the end of 24 h. The serum was rapidly separated and processed for determination of serum uric acid, serum creatinine, blood urea nitrogen (BUN) and serum urea using commercially available kits of Span Diagnostics. Changes in kidney weight were recorded. Three rats per group were sacrificed and both kidneys were isolated from each rat. The kidneys were weighed and processed for histopathological examination.
Histopathological examination of kidney
The kidneys were longitudinally sectioned in two halves and were kept in 10% neutral formalin solution. Both kidneys were processed, embedded in paraffin wax and sections were taken using a microtome. The sections were stained with hematoxylin and eosin and were observed under a computerized light microscope. The data obtained was analyzed using one-way ANOVA followed by Dunnet’s multiple comparison tests. P < 0.01 was considered significant.
RESULTS
Preliminary phytochemical screening
Results of the preliminary phytochemical investigation on Ginkgo biloba ethanolic leaves are shows the presence of steroids, alkaloids, flavonoids, saponins, glycosides etc.
Biochemical parameters
Nephrotoxic animals treated with Ginkgo biloba showed significant decrease in blood urea nitrogen (BUN), serum creatinine, serum uric acid, serum urea and weight of kidney when compared with nephrotoxic group II (Table 1).
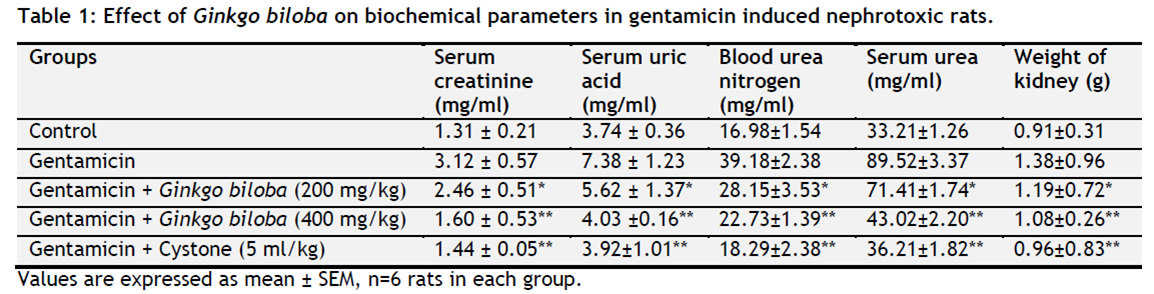 |
Table 1. Effect of Ginkgo biloba on biochemical parameters in gentamicin induced nephrotoxic rats. Click here to view full image |
Histopathology of kidney
Group I kidney section of control group showing normal tubular brush borders, intact glomeruli and Bowman’s capsule. Group II (nephrotoxic) shows the severe tubular necrosis and degranulation. Group III shows showed normal tubular pattern with a mild degree of necrosis, swelling and degranulation. Group IV shows normal tubular pattern, with no degree of necrosis. Group V shows normal tubular brush borders, Bowman’s capsule and intact glomeruli
DISCUSSION
Nephrotoxicity is one of the most common renal problems and it occurs when body is exposed to a drug or toxin (Porter and Bennett, 1981). A number of therapeutic agents can adversely affect the kidney, resulting in acute renal failure, nephritic syndrome and chronic interstitial nephritis and because there is an increasing number of a potent therapeutic drug like aminoglycoside, NSAID’s, antibiotics, chemotherapeutic agents have been added to the therapeutic arsenal in recent years (Hoitsma et al., 1991). Exposure to chemical reagents like ethylene glycol, sodium oxalate, carbon tetrachloride, and heavy metals such as lead, mercury, arsenic and cadmium also induces nephrotoxicity. Prompt recognition of the disease and cessation of responsible drugs, are usually the only necessary therapy (Paller 1990). Nephroprotective agents are the substances which had protective activity against nephrotoxicity. Medicinal plants have been reported, curative properties due to the presence of various complex chemical substances. Early literatures have prescribed various herbs for the cure of renal disorders. Co-administration of various medicinal plants possessing nephroprotective activity along with different nephrotoxic agents which may attenuate its toxicity. The term kidney failure primarily denotes, failure of the excretory function of kidney, leading to retention of nitrogenous waste products of metabolism in the blood (Herfindal and Gourley, 2002). In addition to this, there is a failure of electrolyte balance and regulation of fluid along with endocrine dysfunction. The renal failure is basically categorized into acute and chronic renal failure (Barry et al., 2000).
The kidney disorders are worldwide problem. Despite its frequent occurrence, high morbidity and high mortality, its medical management is currently in adequate, no therapy has successfully prevented the progression of renal diseases, even though newly developed drugs have been used to treat chronic kidney disorders these drugs have often side effects. Therefore, that is an essential research to carry out, the suitable herbal drugs, that could replace the chemical ones. Plants extracts have been used by traditional medical practitioners for the treatment of kidney disorders for centuries. Phenylpropnoids or polyphenolic compounds are a large group of herbal chemical compounds with well-known treat mental and protective effects.
Aminoglycoside antibiotics have been widely used for gram-negative bacterial infections. However, their nephrotoxicity and ototoxicity are the major limitations in clinical use. Among several aminoglycoside antibiotics, the grade of nephrotoxicity has been reported to be in the following order as, neomycin >gentamicin >tobramycin (Hu et al., 1996).
Gentamicin-induced nephrotoxicity occurs in about 15-30% of treated subject is manifested clinically as non-oliguric renal failure, with a slow rise in serum creatinine and hypo-osmolar urinary output developing after several days of treatment (Zaher et al., 2008). Gentamicin is filtered through glomeruli into tubular urine that binds with anionic phospholipids, such as phosphatidylinositol or phospholipdylserine, in brush border membrane of proximal tubular cells reabsorbed actively via pinocytosis process into tubular cells, taken up by lysosomes and thereafter produces phospholipidosis. The drug enters into the cells by adsorptive/receptor mediated endocytosis after binding to acidic phospholipids and megalin and is found essentially in lysosomes. Animals treated with low, therapeutically relevant doses of aminoglycosides show both lysosomal phospholipidosis and apoptosis in proximal tubular cells (Suzuki et al., 1995).
The present thesis entitled “nephroprotective activity on the leaves of Ginkgo biloba against chemical induced toxicity in the experimental rats” deals with the exploration of pharmacological and phytochemical screening of the selected Indian medicinal plant. As already reported that Ginkgo biloba is used in the treatment of high blood pressure, menopause-related cognitive decline, tinnitus, post-stroke recovery, peripheral arterial disease, macular degeneration, or altitude sickness.
CONCLUSION
In the present study, it was observed that treatment with gentamicin induced a significant elevation in the levels of serum urea, creatinine, blood urea nitrogen, serum urea and weight of kidney. However, daily treatment with Ginkgo biloba (200 mg/kg and 400 mg/kg) for 8 days conferred nephroprotection on gentamicin induced rats in a dose dependent fashion offered maximum protection. Further, investigation on the isolation and identification of active components in the leaves lead to chemical entities with potential for clinical use in the prevention and treatment of nephrotoxicity.
CONFLICT OF INTEREST
None declared.
REFERENCES
Abdel Zaher AO, Abdel-Hady RA, Mahmoud MM, Farrag MMY. The potential protective role of alphalipoic acid against acetaminophen-induced hepatic and renal damage. Toxicology. 2008; 243: 261-270.
Atzori C, Bruno A, Chichino G, Bombardelli E, Scaglia M, Ghione M. Activity of bilobalide, a sesquiterpene from Ginkgo biloba, on Pneumocystis carinii. Antimicrobial Agents and Chemotherapy. 1993; 37(7):1492–1496.
Lakshmi BVS, Neelima N, Sudhakar M. Protective effect of Bauhinia purpurea on gentamicin-induced nephrotoxicity in rats. Indian Journal Pharma Sciences. 2009;71(5):551-554.
Barry M, Brenner, Floyd C, Rector. The kidney 6th edition. Vol I, WB. Saunders Company, Philadelphia. 2000; 3-67.
Bombardelli E, Cristoni A, Morazzoni P. Cosmetical uses of Ginkgo extracts and constituents. In: van Beek TA, editor. Ginkgo Biloba. Singapore: Harwood Academic Publishers. 2000; 475–489.
Boonkaew T, Camper ND. Biological activities of Ginkgo extracts. Phytomedicine. 2005;12(4):318–323.
Braquet P. The ginkgolides from Chinese pharmacopeia to a new class of pharmacological agents: the antagonists of platelet activating factor. In: Braquet P, editor. Ginkgolides—Chemistry, Biology, Pharmacologyand Chemical Perspectives. Barcelona, Spain: Prous Science. 1988;1:15–34.
Bridi R, Crossetti FP, Steffen VM, Henriques AT. The antioxidant activity of standardized extract of Ginkgo biloba (EGb 761) in rats. Phytotherapy Research. 2001;15(5):449–451.
Drieu K. Preparation et definition de l’extract de Ginkgo biloba. Presse Medicale. 1986;15(31):1455–1457.
Priyadarsini G, Kumar A, Anbu J, Ashwini Anjana, Ayyasamy S. Nephroprotective activity of decoction of indigofera tinctoria (avuri kudineer) against Cisplatin-induced nephropathy in rats. Internation Journal of Life Science and Pharma Research. 2012; 2(4):56-62.
Herfindal, Gourley. Text book of therapeutic drug and disease management. 7th edition. Charcil Livingstone. London; 2000; 425-36.
Hoitsma AJ, Wetzels JF and Koene RA. Drug induced nephrotoxicity. Aetiology, clinical features and management. Drug Safety. 1991;6 (2):131-147.
Hu JJ, Yoo JSH, Lin M, Wang EJ, Yang CS. Protective effects of diallyl disulfide on acetaminophen-induced toxicities. Food and Chemical Toxicology. 1996;34:963- 969.
Kanchan Gaikwad, Pradeep Dagle, Pushpalata Choughule, YM. Joshi, Vilasrao Kadam. A review on some nephroprotective medicinal plants. International Journal of Pharmaceutical Sciences and Research. 2012; 3,(8):2451-2454.
Maurer K, Ihl R, Dierks T, Frölich L. Clinical efficacy of Ginkgo biloba special extract EGb 761 in dementia of the Alzheimer type. Journal of Psychiatric Research. 1997;31(6):645–655.
Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Archives of Neurology. 1998;55(11):1409–1415.
Paller MS. Drug induced nephropathies. Medical Clinics of North America.1990; 74 (4):909-917.
Pitchumoni SS, Doraiswamy PM. Current status of antioxidant therapy for Alzheimer’s disease. Journal of the American Geriatrics Society. 1998;46(12):1566–1572.
Porter GA and Bennett WM. Nephrotoxic acute renal failure due to common drugs. American Journal of Physiology. 1981; 241(7): F1-F8.
Singh B, Kaur P, Gopichand, Singh RD, Ahuja PS. Biology and chemistry of Ginkgo biloba. Fitoterapia. 2008;79(6):401–418.
Suzuki SS, Takamura J, Yoshida Y, Shinzawa Niwat, Tamatani, SR. Comparison of gentamicin nephrotoxicity between rats and mice. Camparative. Biochemistry and Physiology. 1995;112(1):15-28.
Wadsworth TL, Koop DR. Effects of Ginkgo biloba extract (EGb 761) and quercetin on lipopolysaccharide-induced release of nitric oxide. Chemico-Biological Interactions. 2001;137(1):43–58.









 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.