Lakshmi BVS*, Sudhakar M, Aparna M.
Department of Pharmacology, Malla Reddy College of Pharmacy, Dhulapally Maisammaguda, Secunderabad-500014, AP, India.
ORIGINAL RESEARCH ARTICLE
Volume 2, Issue 1, Jan-April 2014, Page 25-29.
Article history
Received: 04 April 2014
Revised: 20 April 2014
Accepted: 26 April 2014
Early view: 28 April 2014
*Author for correspondence
E-mail: [email protected]
Keywords:
Cadmium
Vitis vinifera
Antioxidants
Nephrotoxicity.
Background: Cadmium (Cd) is a major environmental pollutant and is known for its wide toxic manifestations. The protective effect of concurrent administration of black grapes (Vitis vinifera) on cadmium-induced renal damage was assessed in rats.
Material and methods: In the present investigation cadmium (5 mg/kg) was administered orally for 4 weeks to induce nephrotoxicity in rats.
Results: Tissue damage induced by cadmium was clearly shown by increased activities of renal markers-serum creatinine and blood urea nitrogen (BUN) along with the increased level of lipid peroxidation indices-TBARS, significantly diminished levels of enzymatic antioxidants such as superoxide dismutases (SOD), catalase, and non-enzymatic antioxidants viz. glutathione (GSH). Administration of black grapes extract significantly reversed activities of serum renal markers to their near-normal levels, significantly decreased lipid peroxidation, restored the antioxidant defense levels of in kidney, and produced improvement in hematological parameters when compared to cadmium-treated rats.
Conclusion: The present study suggested that black grapes may be beneficial in ameliorating the cadmium-induced oxidative damage in the kidneys of rats.
INTRODUCTION
Cadmium (Cd) is a relatively rare element that occurs naturally in ores together with zinc, lead and copper or is emitted into the air through the process of volcanic emission. It became commercial in the 20th century due to agricultural and industrial applications (WHO, 2000; Jarup, 2003). Occupational exposure to cadmium, like working with cadmium containing pigments, plastic, glass, metal alloys and electrode material in nickel-cadmium batteries, and non occupational exposure, viz. food, water and cigarette smoke induces uptake of Cd from the environment into the body through pulmonary and enteral pathways (Waisberg et al., 2003). Cadmium absorbed and accumulates mainly in the kidney and liver, and then it is bound to the apoprotein metallothionein (Morales et al., 2006). The intracellular release of cadmium is responsible for the generation of reactive oxygen species, glutathione depletion, lipid peroxidation, protein cross-liking, DNA damage, culminating ultimately in oxidant-induced cell death (Brennan, 1996)
Black grapes (Vitis vinifera.) belonging to the family Vitaceae, is one of the most widely grown fruit crops in the world. Grape juice, jams and raisins are also important commodities in the market of the whole world. Numerous studies focused on the health-promoting and antioxidant effects of grapes. Interest in the health benefits of muscadines has increased due to their high phenolics contents. Most phenolics in muscadines are located in the seeds (Poudel et al., 2008). Gallic acid, catechin and epicatechin are the main phenolics found in muscadine seeds, while ellagic acid and myricetin are the major ones in the skins. Muscadines and grapes (Black grapes), well known for their high levels of antioxidants and polyphenols, have also shown promise as novel antimicrobial agents (Brown et al., 2009), anti-cancer properties (Mertens-Talcott et al., 2006), anti-inflammatory activity (Greenspan, et al., 2006) and antimicrobial activity against Escherichia coli O157:H7 (Kim et al., 2009), antiulcerative, antiarthritic, antioxidant (Yassa et al., 2008), anti-viral, prevent skin aging, scavenge free radicals and inhibit UV-radiation-induced peroxidation activity (Bagachi et al., 1997; Dragsted et al., 1998).
In recent years there has been an augmented interest in the application of antioxidants to medical treatment as information is available linking the development of human diseases to oxidative stress (Aljadi and Kamaruddin, 2004).
Little information is available on protective effect of black grapes against cadmium- induced hepatotoxicity and nephrotoxicity. In the present study an experimental model of rats treated with Cd during four weeks as a model of Cd-induced nephrotoxicity were used. In this model the protective effect of concurrent administration of black grapes on Cd-induced renal damage was assessed and if the protective effect of black grapes is based on its antioxidant properties.
MATERIALS AND METHODS
Collection of plant material
The black grapes were collected from the local market in Ranga Reddy District and the botanical authentication was done by Dr. Ram Chandra Reddy, Head, Department of Botany, Osmania University, Hyderabad, AP, India.
Preparation of extract
The fresh fruits were sliced using a home slicer and the slices obtained were shade-dried, pulverized and passed through a 20-mesh sieve. The dried, coarsely powdered plant material was extracted with hydroalcoholic solvent (60 ml water and 40 ml methanol) using Soxhlet apparatus at a temperature below 60 °C for 24 hours. The solvent was evaporated under vacuum, which gave semisolid mass (yield: 57% w/w) with respect to the dried powder. Oral suspensions containing 400 mg/ml of the hydroalcoholic extract of black grapes were prepared and used for the evaluation of oxidative stress in kidneys of rats.
Chemicals
Cadmium chloride (CdCl2) was purchased from ICN pharmaceutical company (USA).1, 1-diphenyl, 2- picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich, Mumbai. All chemicals for sensitive biochemical assays were obtained from Sigma Chemicals Co., India and Hi-media Chemicals, Mumbai, India. Distilled water was used for biochemical assays. All kits were obtained from Span Diagnostics Ltd., Surat, India.
Animals
Adult male Sprague–Dawley rats (150 ± 10 g body weight) were obtained from the departmental animal facility where they were housed under standard husbandry conditions (25 ± 2 °C temp., 60–70% relative humidity and 12 h photoperiod) with standard rat feed and water ad libitum. Experiments were conducted in accordance with the guidelines set by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India and experimental protocols were approved by the Institutional Animal Ethics Committee (CPCSEA/1217/2008/a).
Experimental design
The rats were divided into four groups of 6 rats each. Rats receiving cadmium chloride were given cadmium chloride 5 mg/kg body weight orally in distilled water. 1/5th of the LD50 dose i.e. 400 mg/kg of the extract was selected for evaluation of cadmium-induced hepatic and renal damage.
• Group I: Normal control group received 0.9 % normal saline by oral route.
• Group II: BGE group received black grape extract 400 mg/kg p.o. daily for 30 days for BGE per se study.
• Group III: Cadmium control group received cadmium chloride 5 mg/kg, p.o. daily for 30 days.
• Group IV: BGE + cadmium group received black grape extract 400 mg/kg, p.o. daily followed by cadmium chloride 5 mg/kg, p.o. daily for 30 days.
On completion of the experimental period, blood was collected in heparinized tubes; serum was isolated to assess various biochemical variables. Animals from all groups (group I-III & IV) were killed on day 31 after initiation of the experiment by cervical decapitation. Tissue samples (kidney) of each animal were immediately processed for the biochemical analysis. All assays were performed with freshly isolated samples. The samples were maintained at −20 °C before performing assays (not longer than 7 days).
Blood biochemical analysis
Blood samples were allowed to stand at room temperature for 30 min and serum was isolated by centrifugation at 1000 × g for 15 min and used for estimation of creatinine and blood urea nitrogen (BUN).
Biochemical assays
Kidneys were minced separately and homogenized (10% w/v) in ice-cold 0.1 M sodium phosphate buffer (pH 7.4). The homogenate was centrifuged at 10,000 rpm for 15–20 min at 4°C twice to get the enzyme fraction. The supernatant was used for biochemical assays.
Estimation of lipid peroxidation (LPO)
LPO was estimated colorimetrically by measuring malondialdehyde (MDA) formation. (Nwanjo and Ojiako, 2005). In brief, 0.1 ml of homogenate was treated with 2 ml of a 1:1:1 ratio of TBA–TCA–HCl (TBA 0.37%, TCA 15%, HCl 0.25 N) and placed in water bath at 65°C for 15 min, cooled, and centrifuged at 5,000 rpm for 10 min at room temperature. The optical density of the clear supernatant was measured at 535 nm against a reference blank. The MDA formed was calculated by using the molar extinction coefficient of thiobarbituric acid reactants (TBARS; 1.56×105 l/mole cm−1). The product of LPO was expressed as nmol of MDA formed per g of tissue.
Estimation of superoxide dismutase (SOD)
Renal SOD activity was assayed according to the method of Marklund and Marklund (1974). For the control, 0.1 ml of 20 mM pyrogallol solution was added to 2.9 ml of Tris buffer and mixed, and reading was taken at 420 nm after 1.5 and 3.5 min. The absorbance difference for 2 min was recorded and the concentration of pyrogallol was adjusted in such a way that the rate in change of absorbance per 2 min was approximately 0.020–0.023 optical density units.
Kidney extract (200 µl) was treated with 10 µl of 25% triton X-100 and kept at 4°C for 30 min. To 2.8 ml of Tris buffer, 0.1 ml of treated sample was added and mixed, and the reaction was started by adding 0.1 ml of adjusted pyrogallol solution (as for control). Reading was taken at 420 nm after 1.5 and 3.5 min and the difference in absorbance was recorded. The enzyme activity was expressed as U/ml of extract and 1 U of enzyme is defined as the enzyme activity that inhibits auto-oxidation of pyrogallol by 50%.
Estimation of catalase (CAT)
Catalase (CAT) activity was estimated following the method of Aebi (1993). The homogenate (100 µl) was treated with ethanol (10 µl) and placed on an ice bath for 30 min. To this, 10 µl of 25% triton X-100 was added and again kept for 30 min on ice. To 200 µl phosphate buffer (0.1 M), 50 µl of treated kidney homogenate and 250 µl of 0.066 M H2O2 (prepared in 0.1 M phosphate buffer, pH 7.0) were added in a cuvette. The reduction in optical density was measured at 240 nm for 60 seconds. The molar extinction coefficient of 43.6 cm−1 was used to determine CAT activity. One unit of activity is equal to the moles of H2O2degraded/min/mg protein.
Estimation of glutathione (GSH)
Reduced glutathione (GSH) was determined by the method of Ellman (1959). In brief, 1 ml of supernatant was taken after precipitating 0.5 ml of kidney homogenate with 2 ml of 5% TCA. To this, 0.5 ml of Ellman’s reagent (0.0198% DTNB in 1% sodium citrate) and 3 ml of phosphate buffer (1 M, pH 8.0) was added. The color developed was read at 412 nm. Reduced GSH concentration is measured by using a drawn standard curve and was expressed as mg/g of tissue.
Statistical analysis
The experimental results were expressed as the mean ± SEM with 6 rats in each group. The intergroup variation between various groups were analyzed statistically using one-way analysis of variance (ANOVA) using the Graph Pad Prism version 5.0, followed by Dunnett’s multiple comparison test (DMCT). Results were considered statistically significant when P < 0.05.
RESULTS
Effect of black grapes on serum parameters on BUN and creatinine levels in cadmium chloride-induced toxicity
Cadmium exposure produced significant increase in BUN (P < 0.01) and creatinine (P < 0.001) levels in cadmium control group when compared to normal control group. Pretreatment of hydroalcoholic extract of black grapes before cadmium exposure showed significant decrease (P < 0.05) in BUN and creatinine levels in BGE + CD when compared to cadmium control group (Fig. 1).
Effect of black grapes on kidney weight in cadmium chloride-induced toxicity
Figure 2 showed the changes in the kidney weights of treated rats and control. As shown in the figure, cadmium chloride induced a significant (P < 0.01) reduction on the average organs weight (kidney). However, post-treatment with black grapes improved the kidney’s weight significantly (P < 0.05).
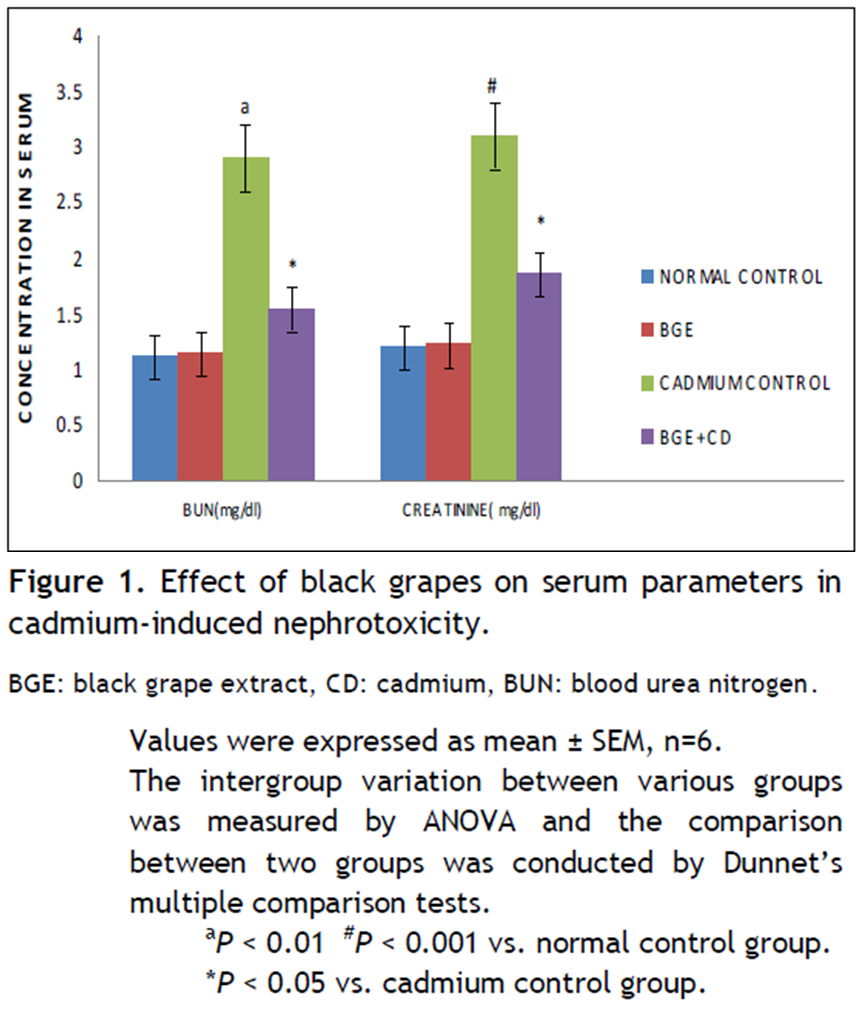 |
Figure 1. Effect of black grapes on serum parameters in cadmium-induced nephrotoxicity. Click here to view full image |
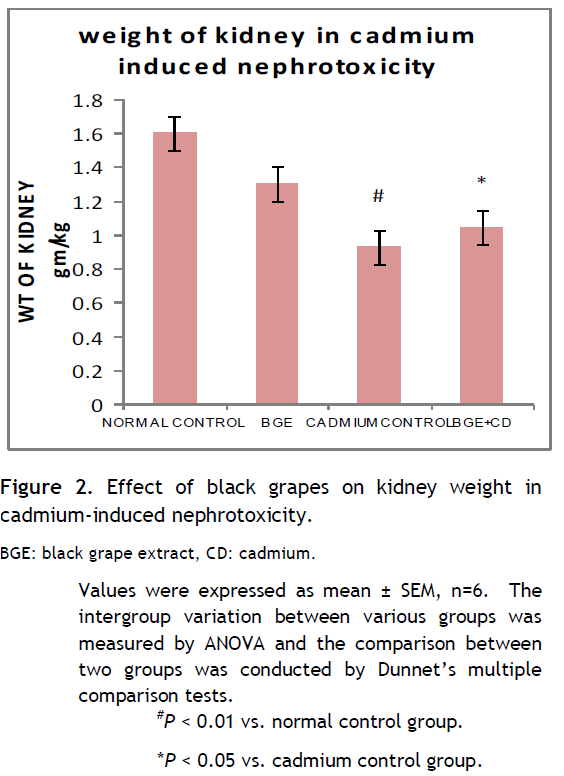 |
Table 2. Effect of black grapes on kidney weight in cadmium-induced nephrotoxicity. Click here to view full image |
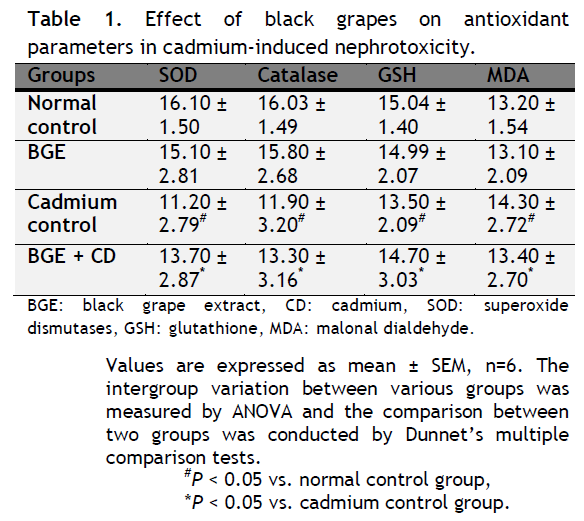 |
Table 1. Effect of black grapes on antioxidant parameters in cadmium-induced nephrotoxicity. Click here to view full image |
DISCUSSION
Available literature indicates that no previous studies have been done to evaluate the antioxidant capacity of black grapes and its protective effect against cadmium intoxication. The mechanisms of cadmium-induced damage include the production of free radicals that alter mitochondrial activity and genetic information (Patrick, 2003; De Burbure et al., 2006). Therefore, some authors have postulated that antioxidants should be one of the important components of an effective treatment of cadmium poisoning (El-Demerdash et al., 2004).
The present study concentrates on the possible protective effect of black grapes on oxidative damage generated by cadmium induced nephrotoxicity. Kidney function tests were done for different studied groups to assess their status. Biochemical analysis was done for oxidative stress indices such as lipid peroxidase level. The activity of antioxidants was measured e.g. reduced glutathione, (GSH), superoxide dismutase (SOD) and catalase (CAT) because these antioxidants are the commonest to be affected by cadmium toxicity (Jurezuk et al., 2004).
The levels of creatinine and urea were significantly increased after cadmium treatment compared to the control group indicating the impairment in the kidney function. Similar observation was obtained by Novelli et al., (1998). In fact, urea is the first acute renal marker which increases when the kidney suffers any kind of injury. Otherwise, creatinine is the most trustable renal marker and increase only when the majority of renal function is lost (Borge et al., 2005). The changes in urea and creatinine level in the present study concluded the severe injured effect of CdCl2 on kidney. Moreover, in the present study black grapes co-administration with cadmium significantly ameliorated the increased levels of creatinine and urea.
In the current study, lipid peroxidation level was significantly elevated in serum, kidney tissues of rats treated with cadmium compared to control group thus suggesting increased oxidative stress. These results were supported by Manca et al., (1991); Hassoun and Stohs (1996) and Jurezuk et al., (2004) who reported that LPO is an early and sensitive consequence of Cd exposure.
Cd was excessively accumulated in kidney. In animals exposed to Cd via oral routes, the kidney is by far the primary organ affected adversely by Cd. Some investigators have suggested that, under conditions of chronic exposure to Cd, complexes of Cd-metallothionein (formed in hepatocytes in response to the uptake of Cd) are released from necrotic hepatocytes and are delivered (via systemic circulation) to the kidneys, where it appears that they are taken up and induce proximal tubular injury and death.
In the present study, the elevation in the free radicals (LPO) induced by cadmium alone was very significantly decreased in the presence of black grapes. This means that black grapes minimized the toxic effect of cadmium via its antioxidant activity. These results are in line with the view held by Yassa et al., (2008) who confirmed the role of black grapes as an antioxidant agent in blood.
In agreement with a previous study, the level of GSH was significantly decreased in the kidney extracts of cadmium treated group compared to the control group. This decrease in GSH levels may be due to its consumption in the prevention of free radical-mediated lipid peroxidation (Demopoulos, 1973). GSH may be consumed in the detoxification of heavy metals (Kim et al., 1998). Furthermore, it has been suggested that the decrease in GSH levels upon cadmium exposure might impair the degradation of lipid peroxides, thereby leading to its accumulation in the target organs. In controversy to the current results, Kamiyama et al., (1995) reported an increase in GSH level in kidney tissues after Cd injection which could be explained as a protective mechanism.
Epidemiological studies have revealed that cadmium is one of the most toxic of the heavy metals to humans; 70% of the ultrafiltered cadmium is taken up, largely by the proximal tubules of the kidney and is accumulated mainly in kidney cortex leading to proximal tubule lesions (Lars, 2002). These findings are in agreement with the current study. The nephrotoxicity of Cd has been extensively studied in various experimental models. Recent papers show that tubular damage may appear at lower levels of Cd exposure than previously anticipated (Jarup et al., 2000).
In the present study, CdCl2 exposure increased LPO levels in tissues with alterations in antioxidant defenses (SOD, CAT and GSH). Thus it may be possible that oxidative stress and disturbance in antioxidant defenses were the causes for kidney damage induced by CdCl2 in this experimental model.
On hypothesis to explain the beneficial effects of black grapes in ameliorating biochemical parameters is that black grapes may contains flavonoids, catalase and phenolic compounds including resvaratrol, anthocyanins. All of which work together to provide a synergistic antioxidant effect, scavenging and eliminating free radicals (Johnston et al., 2005).
CONCLUSION
The present study demonstrated that black grapes administered in combination with cadmium minimized its hazards. Black grapes can protect against oxidative stress induced by cadmium, by lowering the free radicals and increased the levels of antioxidants. Further studies are required to recommend the use of black grapes and its therapeutic potential in human.
ACKNOWLEDGEMENT
The authors are thankful to the authorities of Malla Reddy College of Pharmacy, Secunderabad, for providing support to this study.
CONFLICT OF INTEREST
None declared.
REFERENCES
Aebi HE. Catalase. In: Bergmeyer HU, Bergmeyer J, Grabl M, editors. Methods of Enzymatic Analysis. Weinheim: Velag Chemie Gmbh, pp 273–286, 1993.
Aljadi A, Kamaruddin M. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 85, 513-518, 2004.
Bagachi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamin C and E, and a grape seed proanthocyanidins extract in vitro. Res Common Mol Pathol Pharmacol. 95, 179, 1997.
Borges LP, Borges VC, Moro AV, Nogueira CW, Rocha JB, Zeni G. Protective effect of diphenyldiselenide on acute liver damage induced by 2- Nitropropane in rats. Toxicology. 210, 1-8, 2005.
Brennan RJ. Cadmium is an inducer of oxidative stress in yeast. Mutat Res. 356, 171-178, 1996.
Brown JC, Huang G, Haley-Zitlin V, Jiang X. Antibacterial effects of grape extracts on Helicobacter pylori. Appl Environ Microbiol. 75, 848-852, 2009.
Demopoulos H. Control of free radicals in biological system. Fed Proc. 32, 1903-1908, 1973.
Dragsted LO. Natural antioxidants in chemoprevention. Arch Toxicol Suppl. 20, 209, 1998.
El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and B-carotene. Food Chem Toxic. 42, 1563-1571, 2004.
Elizabeth AM, Rosalyn DM, Jennifer AM, Rebecca RW, Beth AA. Environmental cadmium levels increase phytochelatin and glutathione in lettuce grown in a chelator-buffered nutrient solution. Environ Qual. 32, 1356-1364, 2003.
Ellman GC. Tissue sulfhydryl groups. Arch Biochem Biophys. 82, 70–77, 1959.
Greenspan P, Bauer JD, Pollock SH, Gangemi JD, Mayer EP, Ghaffar A, Hargrove JL, Hartle DK. Antiinflammatory properties of the muscadine grape (Vitis rotundifolia). J Agric Food Chem. 253, 8481-8484, 2005.
Hassoun EA, Stohs SJ. Cadmium-induced production of superoxide anion and nitric oxide, DNA single strands breaks and lactate dehydrogenase leakage in J774A.1 cell cultures. Toxicology. 112, 219-226, 1996.
Jarup L, Hellstrom L, Alfven T, Carlsson MD, Grubb A, Persson B, Pettersson C, Spang G, Schutz A, Elinder CG. Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup Environ Med. 57, 668-672, 2000.
Johnston J, Sepe H, Miano C, Brannan R, Alderton A. Honey inhibits lipid oxidation in ready-to-eat ground beef patties. Meat Sci. 70, 627-631, 2005.
Jurezuk M, Brzoska J, MoniuszkoJakoniuk M, Galazyn-Sidorczuk, Kulikowska-Karpinska E. Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food Chem Toxicol, 42, 429-438, 2004.
Kamiyama T, Miyakawa H, Li JP, Akiba T, Liu JH, Marumo F, Sato C. Effects of one year cad-mium exposure in livers and kidneys and their relation to glutathione levels. Res Commun Mol Pathol Pharmacol, 88, 177-186, 1995.
Kim CY, Lee MJ, Lee SM, Lee WC, Kim JS. Effect of melatonin on cadmium induced hepatotoxicity in male rats. Tohoku J Exp Med, 186, 205-213, 1998.
Lamberg SL, Rothstein R. Laboratory Manual of Hematology and Urinalysis. Avi Publishing Company, Inc: West Port Connecticut, USSR, 1977.
Lars, J. Cadmium overload and toxicity. Nephrol Dial Transplant. 17, 35-39, 2002.
Manca D, Ricard AC, Trottier B, Chevalier G. Studies on lipid peroxidation in rat tissues following administration of low and moderate doses of cadmium chloride. Toxicology. 67, 303-323, 1991.
Marklund S, Marklund G. Involvement of superoxide anion radical in the autooxidation of pyrogallol and convenient assay for SOD. Eur J Biochem. 47, 469–474, 1974.
Mertens-Talcott SU, Zadezensky I, De Castro WV, Derendorf H, Butterweck V. Grapefruit-drug interactions: can interactions with drugs be avoided? J Clin Pharmacol, 46, 1390-1416, 2006.
Morales A, Vicente C, Santiag J, Egido J, Mayoral P, Arevalo M, Fernandez M, Lopez-Novoa J, Perez F. Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem Toxicol. 44, 2092-2100, 2006.
Novelli E, Vieira E, Rodrigues N, Ribas B. Risk assessment of cadmium toxicity on hepatic and renal tissues of rats. Environ Res. 79, 102-105, 1998.
Nwanjo HU, Ojiako OA. Effect of vitamins E and C on exercise induced oxidative stress. Global J Pure Appl Sci. 12, 199–202, 2005.
Partrick L. Toxic metals and antioxidants: Part II. The role of antioxidants in arsenic and cadmium toxicity. Altern Med Rev. 8, 106-128, 2003.
Poudel PR, Tamura H, Kataoka I, Mochioka R. Phenolic compounds and antioxidant activities of skins and seeds of five wild grapes and two hybrids native to Japan. J Food Comp Anal. 21, 622-625, 2008.
Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 192, 195-117, 2003.
World Health organization (WHO) cadmium air quality quide lines. 2nd edition, WHO, regional office for Europe: Copenhagen Denmark, 2000.
Yassa N, Beni HR, Hadjiakhoondi A, Free radical scavenging and lipid peroxidation activity of the shahani black grape. Pak J Biol Sci. 11, 2513-2516, 2008.









 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.