Athar Parvez Ansari1*, Zaheer Ahmed N2, Mohammad Sheeraz3
1,2Regional Research Institute of Unani Medicine (CCRUM, Ministry of AYUSH, Govt of India), Royapuram, Chennai–600 013 (TN), India.
3Regional Research Institute of Unani Medicine (CCRUM, Ministry of AYUSH, Govt of India), Bhadrak–756 100 (Odisha), India.
REVIEW ARTICLE
Volume 4, Issue 1, Page 22-28, January-April 2016.
Article history
Received: 12 January 2016
Revised: 26 January 2016
Accepted: 13 February 2016
Early view: 25 February 2016
*Author for correspondence
E-mail: [email protected]
The compounding of Unani drugs is profoundly rooted in history. The earliest preparations i.e., Sufoof (Powder) and Tiryaq (Anti-dote) were prepared by Arastu (Aristotle) and Indrumakhas I (Andromachus I) respectively. Some of the preparations like Habb-e-Ayarij, Amroosiya, Basaliqoon and Ointment etc., were introduced by Buqrat (Hippocrates). But the large numbers of dosage forms were prepared by Jalinus (Galen) who propounded that the patient’s body would pull out of a complex prescription the substances that it needed to restore its humoral balance. Hence, he is considered as the father of polyherbal pharmaceutical preparations. One of the prime factors for not reaching Unani System of Medicine to the public at large is the stagnation in drug dosage forms. Nearly 90 dosage forms are recorded in Unani pharmaceutical books, of them only 1/3rd is used in present era. Most of the dosage forms remain only an ornament of books because of varied reasons viz. time consuming preparations, not easy to carry, large bulk of doses, not so attractive, unsterilized method of handling, delayed action, high sugar content etc. Thus, it is highly imperative to modify the Unani drug dosage forms. Modifications should be done in all three phases of pharmaceutical drugs i.e., before, during and after preparations. Apart from this, several dosage forms which are not used may be changed into sub lingual, chewable and dispersible tablets, lozenges, drops, inhalers, suppositories, pessaries, cosmetics, enemas, etc. Unless and until afore said measures are taken on priority basis, the survival of the system will remain a quagmire.
Keywords: Unani drugs; dosage forms; ashkale advia; modification.
INTRODUCTION
The exact time of origin of the earliest dosage forms is lost in the mists of history. It can be safely assumed that primitive man took parts of plants including leaves, stems, roots and berries internally for a range of symptoms. A variety of plant and animal materials would undoubtedly have also been applied externally to aid the healing of wounds (Anonymous, 2015).
The earliest comprehensive Materia medica was written at the time of King Assurbanipal 2000 years B.C. containing approximately 250 vegetable and 120 mineral drugs. Around 1500 B.C., the famous Egyptian Papyrus Ebers is the best known pharmaceutical record containing 800 prescriptions using 700 drugs drawn from plants, animals and minerals origin (Jokhab, 2015). The ancient Greeks and Romans used a number of dosage forms, many of which can be recognized today; these included ointments, oils, powders, pills, pessaries, gargles and eye lotions etc (Anonymous, 2015).
Unani Medicine claims earliest preparations viz; Sufoof (Powder) (Rehman, 1991; Bari, 2003; Said, 1997) and Tiryaq (Anti-dote) were prepared by Arastu (Aristotle) and Magneeus Felsoof respectively. Indrumakhas I (Andromachus I) modified Tiryaq, mixed fleshes of snakes into Tiryaq and given name Tiryaq-e-Farooq (Ibn Abbas, 2010). Some of the preparations like Ayarij Feeqra (Arzani, 1998) Amroosiya, Basaliqoon (Arzani, 1998; Said, 1997), and ointment etc, were introduced by Buqrat (Hippocrates). (Rehman, 1991) But the large number of dosage forms were prepared by Jalinus (Galen) who propounded that the patient’s body would pull out of a complex prescription the substances that it needed to restore its humoral balance. Jalinus practiced and taught both pharmacy and medicine in Rome; his principles of preparing and compounding drugs ruled the Western world for 1,500 years; and his name still is associated with that class of pharmaceuticals compounded by mechanical means-Galenicals. He was the originator of the formula for a cold cream, essentially similar to that known today. Many procedures Galen originated have their counterparts in today’s modern compounding laboratories (George, 1965). Hence, he is considered as the father of polyherbal pharmaceutical preparations. Barshasha, Laooq (Linctus) and Huqna (Enema) were also introduced by him in pharmaceutics (Rehman, 1991; Bari, 2003).
After Jalinus many Unani physicians introduced several dosage forms viz; Jawarish and Gulqand were invented by Iranian physicians. Hamul (Pessary), Farzaja (Vaginal pessary) and Fateela (Bougie) were introduced by Bukhtishu. Zimad (Paste) was prepared by Egyptian physicians while Arq (Aqueous) (Rehman, 1991) and Kushta (Calx) were made by Arab physicians. It is claimed that Majun (Confection) was invented by Hermes (Rehman, 1991; Bari, 2003). Sharbat (Syrup) was introduced by Feesagorus (Pythagoras). Khameera (Fermented confection) was invented by Hakims (Unani physicians) of Mughal period (Table 1) (Rehman, 1991; Said, 1997).
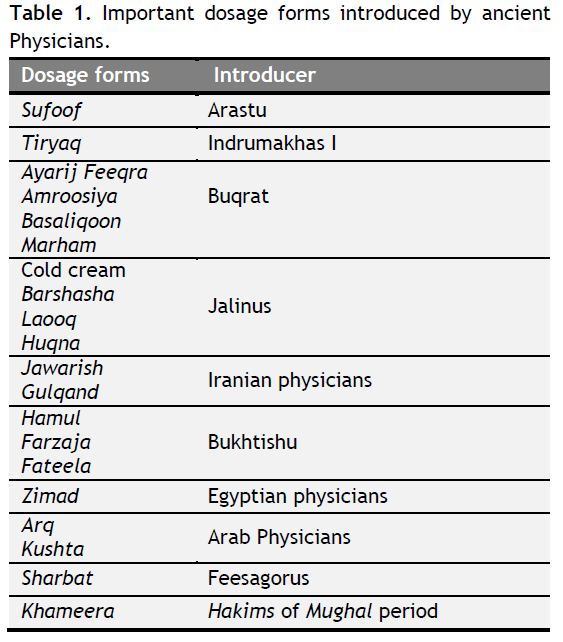 |
Table 1. Important dosage forms introduced by ancient Physicians. Click here to view full image |
Apart from dosage forms individual formulations were also prepared by different Unani physicians viz; Habb-e-Qoqaya and Majun Sara were first prepared by Jalinus, Habb-e-Zahab introduced by Ibn Sina (Avicenna), Majun Baladuri was prepared by Zakariya Razi (Rhazes), Majun Flasafa was introduced by Indromakhas II (Andromachus II) while Majun Najah is ascribed to Hermes, and to Jalinus as claimed by some (Table 2) (Arzani, 1998).
Need of dosage forms
Unani Medicine defines Ashkal-e-Advia as “drug is used either singly or in combination but it is never used in its original form, hence the form has to be modified” (Qureshi, 1995). Unani physicians have difference of opinion over the use of compound formulations; many of them advocate single drug is best for treatment of diseases. Razi quoted “whenever possible, always use single drugs for treatment of diseases; compound formulations may be used only in compelling situation’’. Ibn Sina stated that “tested drugs are better than untested drugs and single drugs should be used in single diseases than compound formulations”. But sometimes, single drugs are not sufficient to cure a disease. According to some Unani physicians this principle of treatment has limitations, may be accepted only in certain conditions. Majusi (Haly Abbas) said “all diseases could not be cured by single drugs because the temperaments of disease and drug may vary”. Several Unani physicians are of the opinion that treatment of diseases with single drugs is confined to single diseases” (Qureshi, 1995).
 |
Table 2. Individual dosage forms introduced by ancient physicians. Click here to view full image |
However, after much deliberation, Unani physicians advocated treatment by compound drugs which are best because of varied reasons viz; Islah-e-Advia ke liye (for correction of drugs), Izafa-e-Quwat ke liye (to increase potency), Tazeef-e-Amal ke liye (to decrease action), Sura’t-e-Nufooz ke vaste (to increase absorption), Ibta-e-Nufooz ke vaste (to decrease absorption), Amraz-e-Murakkaba ke ilaj ke liye (for treatment of compound diseases), Tahaffuz-e-Advia ke liye (for preservation of drugs), Dawa ki miqdar-e-Khurak ko badhana (to increase the bulk of drug) and Dawa-e-Mufrad ki taseer-e-Kulli ko juza’i banana (to decrease the effect of drug in whole body) (Wadud, 2004; Qureshi, 1995; Ibn Sina, 2006; Ibnul Quf, 1986).
Today, several dosage forms have been modified in Conventional Medicine as per need viz; to provide optimal drug action from topical administration sites (transdermal patches), to provide for insertion of a drug into one of the body’s orifices (rectal or vaginal suppositories), to protect the drug substance from the destructive influences of atmospheric oxygen or humidity (coated tablets, sealed ampoules), to protect the drug substance from the destructive influence of gastric acid after oral administration (enteric-coated tablets) (Chaudhary et al, 2013). Even many modern dosage forms are referred to in the Ebers papyrus viz; gargles, snuffs, inhalations, suppositories etc. (Jokhab, 2015).
Types of dosage forms
The term pharmacopoeia was coined by Dr. Foes in 1561 A.D. Pharmacopoeia is a book containing directions for the identification of sample and the preparation of compound medicines and published by the authority of a government or a medical or pharmaceutical society (World Health Organization, 2013). Similarly the term Qarabadeen / Anqarabadeen / Aqrabadeen was derived from an Arabic word Ankarabadeen. Probably Ali Gilani was the first to use and gave this term, meaning ‘Advia murakkaba ka dafter’ (office of compound drugs) and Arzani quoted the meaning of Qarabadeen is ‘Advia murakkaba’ (compound drugs) in Qarabadeen-e-Qadri. (Arzani,1998). In India, fourteen Qarabadeen are approved by Ministry of AYUSH, Govt. of India apart from other several pharmacopoeias. These are Qarabadeen-e-Qadri by Akbar Arzani, Qarabadeen-e-Kabeer by Mohammad Husain Khan, Qarabadeen-e-Azam by Azam Khan, Qarabadeen-e-Jadeed by Abdul Hafeez, Elaj-ul-Amraz by Shareef Khan, Bayaz-e-Kabeer Volume II and Kitab al-Taklees by Kabeerudeen, Miftah-ul-Khazain by Kareem Baksh, Ma’dan al-Akseer by Firozuddin, Makhzan-ul-Murakkabat by Ghulam Jeelani, Al Qarabadeen-e-Jadeed by Kabeeruddin, Kitab al-Taklees by Abdul Hafeez, National Formulary of Unani Medicine and Unani Pharmacopoeia (Lohar, YNM).
Mostly all Unani pharmacopoeias broadly classify Ashkal-e-Advia into four broad categories: (1) Jamid Advia (Solid dosage forms), (2) Neem Jamid Advia (Semi-solid dosage forms), (3) Saiyyal Advia (Liquid dosage forms), (4) Bukhari Advia (Gaseous dosage forms) (Table 3) (Wadud, 2004; Qureshi, 1995; Ibn Sina, 2006; Chaudhary et al., 2013; Ghani, 2010).
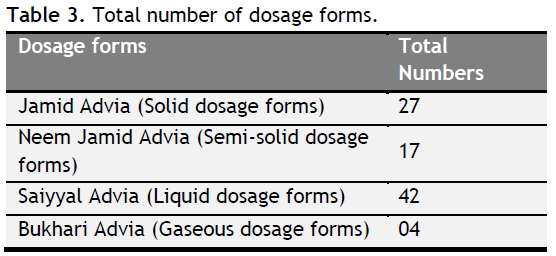 |
Table 3. Total number of dosage forms.. Click here to view full image |
Jamid advia (Solid dosage forms): Hab (Pill), Qurs (Tablet), Sufoof (Powder), Shiyaf (Suppository), Hamool (Pessary), Farzaja (Vaginal pessary) Fateela (Bougie), Sanoon (Tooth powder), Mazoogh (Masticator), Burood (Eye dusting powder), Kohl (Collyrium), Zaroor (Dusting powder), Usara, Nafookh (Insuflation), Atoos (Errhine), Ghaza (Face powder), Ghalia (Perfumed powder), Ubtana, Lazooq (Band-aid), Norah (Hair remover), Murabba (Preserver), Gulqand, Rub (Extract), Halva (Sweet), Mishtri, Damam and Argaja (Wadud, 2004; Qureshi, 1995; Ibn Sina, 2006; Ghani, 2010; Jalaluddin, 2006; Ali, 2006; Khan, 2006; Khan, 2005).
Neem jamid advia (Semi-solid dosage forms): Majoon (Confection), Itrifal, Jawarish, Anushdaru, Khameera (Fermented confection), Laooq (Linctus), Laboob, Dawa-ul-Misk, Yaqooti, Bershasha, Tiryaq (Anti-dote), Zarooni, Usara, Marham (Ointment), Qeruti (Poultice), Zimad (Paste) and Latookh (Wadud, 2004; Qureshi, 1995; Ibn Sina, 2006; Ghani, 2010; Jalaluddin, 2006; Ali, 2006; Khan, 2006; Khan, 2005).
However, Itrifalat, Anushdaru, Jawarishat, Majunat, Khameerajat, Laooqat, Labubat, Muffarehat and Dawa-ul-Misk could be classified as Majoon, but have been classified separately because of their special characteristics (Rehman, 1991; Said, 1997).
Saiyyal advia (Liquid dosage forms): Ma-ul-Jubn (Whey), Ma-ul-Asl (Hydromel), Ma-ul-Lahem (Mutton soup), Ma-ush-Sha’eer (Barley water),Ma-ul-Buqool (Vegetable juice), Ma-ul-Favakiha (Fruit juice), Rooh (Essence), Sharbat (Syrup), Sikanjbeen, Nabeez, Joshanda (Decoction), Khisanda (Infusion), Zulal, Haleeb va mazeej (Mixture), Sharab (Wine), Sirka (Vinegar), Aabkama, Mehlool (Solution), Nutool (Irrigation), Sakub, Ghusool (Washing lotion), Pashoya (Foot bath), Nazooh (Spray), Tila (Liniment), Dalook (Rubbing agent), Dohan (Oil), Khizab (Hair dye), Sabigh (Skin dye), Mazmaza (Mouth wash), Gharghara (Gargle), Wajoor (Throat drop), Saoot (Nasal drop), Nashooq (Snuff), Zarooq (Syringing), Aabzan (Sitz bath), Huqna (Enema), Masooh, Marauwakh, Lua’bat (Mucilage), Dayaqooza, Arq (Distillate), Qutoor (Eye drop) (Wadud, 2004; Qureshi, 1995; Ibn Sina, 2006; Ghani, 2010; Jalaluddin, 2006; Ali, 2006; Khan, 2006; Khan, 2005).
Bukhari advia (Gaseous dosage forms): Bukhoor / Dhooni (Fumigation), Inkibab/Bhapara (Vapour bath), Lakhlakha (Inhalation) and Shamoom (Olfaction) (Wadud, 2004; Qureshi, 1995; Ibn Sina, 2006; Ghani, 2010; Jalaluddin, 2006; Ali, 2006; Khan, 2006; Khan, 2005).
Need of modification in dosage forms
At present, nearly 90 dosage forms are recorded in different Unani pharmacopoeias (Arzani, 1998; Said, 1997; Ibn Sina, 2006; Ghani, 2010; Kabeeruddin, 1935; Hafeez, 2005), among them only 1/3rd is in vogue. Most of the dosage forms remain only an ornament of books because of varied reasons viz. time consuming preparations, uneasy to carry, large bulk of dosage, unattractiveness, unsterilized method of handling, delayed action, high sugar content etc. Thus, it is highly imperative to modify the Unani drug dosage forms. Modifications should be done in all three phases of pharmaceutical drugs i.e., before, during and after preparations.
Modification in single drugs before compounding
All single drugs which are used in preparations should be identified by Pharmacognostical methods. Those single drugs which are not yet classified scientifically must be classified on the basis of their taxonomy rather than alphabetical classification because this classification is only suitable for quick reference. Mufrad advia (single drugs) must be collected by qualified persons who are aware about the medicinal benefits and appropriate season of collection of drugs. Foreign matter found in a sample of herbal drug should not go beyond the limit set in national or international pharmacopoeias (World Health Organization, 2007). All collected single drugs must be stored properly as per guidelines of Unani pharmacopoeias (Qureshi, 1995; Ibnul Quf, 1986). Cultivation of several species of Unani drugs may be taken as priority basis because cultivated drugs may have good concentrations of active constituents (Kokate, 2009). In this regard, a cohesive approach is essential to co-ordinate with different stake holders like medicinal plants board, state agriculture and forest departments etc, to procure quality of drugs.
The Doses of individual drugs may be standardized with the help of pre-clinical studies. The doses of various single Unani drugs vary in different books. A dose range of each drug may be given. The World Health Organization emphasizes more on safety aspect of a drug rather than efficacy. Hence, toxicological studies are imperative. All single drugs must be tested in animals for their toxicity. Majusi also suggested for animal study of Unani drugs (Ibn Abbas, 2010). Several Unani drugs are contradictory, have different species and few drugs are not easily available and some are extinct. These matters should be sorted out.
Modification in processing
The premises where the preparation is done should be according to the Good Manufacturing Practices (GMP) guidelines for Ayurveda, Siddha and Unani Medicines. All instruments should be labeled by ISI (Indian Standards Institute). Sterilization facility should be available for premises, instruments, containers and individuals. All processes should be done by using modern machineries to reduce man power, time and to increase production at short intervals. All preparations should be prepared with the help of old as well modern parameters, methods and techniques. Few old parameters may be modified viz; instead of taar ka qiwam, viscosity of drugs may be tested. Classical number of sieve may be replaced by British standard of sieve to get uniform particle size of powder drugs.
Modification in dosage forms
Powder
Particle size of the drug plays a major role in the dissolution rate of the drugs. Almost all the formulations are prepared by powder drugs. For high potency drugs, it can be advantageous to have small particle sizes to maximize the number of particles in order to allow adequate mixing and dose uniformity. Since small particles have greater significance (Lund, 2009). In Unani Medicine, Attiba (Unani physicians) have mentioned powder of Jawarish should be coarse (durdura) because these preparations are required to stay in gastrointestinal tract for long time for gradual action (Kabeeruddin, 1935; Hafeez, 2005). Sometimes the powder should be fine in case of Majun and Kushta for quick absorption.
Attiba have also mentioned the size of mesh to confirm the particle size like 40, 60, 80, 100 number of meshes (Said, 1997) which were also used by westerners in 20th Century A.D. but now the method is obsolete and ‘British Standard of sieve size 410: 1986’is in vogue. The old method represents the number of openings per linear inch of mesh. But the British Pharmacopoeia defined in terms of particle size as determined by microscopy (Lund, 2009). The following terms are used:
Coarse powder: All the particles of powder pass through a sieve with a nominal mesh aperture of 1700 micrometers.
Moderately coarse powder: All the particles of powder pass through a sieve with a nominal mesh aperture of 710 micrometers.
Moderately fine powder: All the particles of powder pass through a sieve with a nominal mesh aperture of 335 micrometers.
Fine powder: All the particles of powder pass through a sieve with a nominal mesh aperture of 180 micrometers.
Very fine powder: All the particles of powder pass through a sieve with a nominal mesh aperture of 125 micrometers.
Micro fine powder: All the particles of powder pass through a sieve with a nominal mesh aperture of 45 micrometers.
Superfine powder: All the particles of powder pass through a sieve with a nominal mesh aperture of 10 micrometers (British Standard 410: 1986) (Lund, 2009).
Qurs (Tablet)
According to British pharmacopoeia tablet was invented by Arab physicians (Lund, 2009). The tablet should be stable. Stability of Unani tablets is very weak, after some time of preparation they easily break down. For better stability, diluents, granulating agent, lubricant, glidant and disintegrant may be taken of good quality. All processes of making tablets viz.; mixing, wetting, granulation, drying, sieving and compression should be followed in systematic manner for better stability and disintegration.
Sublingual tablets
Conventional Medicine is preparing lipid soluble tablets and pills for sublingual use. These types of drugs are readily absorbed through the sublingual veins and immediately reach the circulation. They may be helpful in certain emergencies like Ischemic Heart Diseases and Hypertension etc. (Tripathi, 2003). The same can be imbibed for Unani drugs also to meet emergencies.
Lozenges
Lozenges are large tablets that are intended to stay in the mouth for relatively long periods (10-15min), while they dissolve or erode (Lund, 2009). In Unani Medicine, only Sualeen is available as lozenges. Unani Medicine can prepare more lozenges for antibacterial and anesthetic local effect for throat and mouth diseases.
Chewable tablets
In contrast to lozenges, chewable tablets are designed to be broken down rapidly in the buccal cavity by the action of the teeth (Lund, 2009; Gopal et al., 2012). Chewable tablets are preferably given to children, old and bedridden patients, who have difficulty in swallowing or dislike swallowing but at the same time palatable (Gopal et al., 2012).
Dispersible tablets
Dispersible tablet is defined as uncoated or film coated tablet intended to be dispersed in water before administration giving a homogeneous dispersion. Typically, a dispersible tablet is dispersed in about 5-15 ml of water and the resulting dispersion is administered to the patient. However, they can also be placed directly on the tongue and sucked. The dispersion properties of dispersible tablets can be facilitated by the inclusion of an acid/ base couple in which the base liberates carbon dioxide when the components of the couple are dissolved in water (Amarnath et al., 2014).
Bulk density of Majoon, Itrifal, Khameera, Laooq and Jawarish should be determined by bulk density meter. It will be helpful in the identification of an adulterant.
Viscosity of those dosage forms and Sharbat, Joshanda, Khisanda and other liquid form drugs should also be determined by viscometer. It will also be helpful in the identification of an adulterant.
pH of those dosage forms should also be determined by pH meter. Other physico-chemical standardization such as moisture content, ash value and qualitative analysis of phyto-constituents may also be analyzed (Lateef et al., 2013; Meena et al., 2010).
Lazooq (Band-aid)
The traditional method to prepare this dosage form is fine powder of drugs mixed to white part of egg or other mucilaginous substances then spread over already sieved paper and apply the paper on affected area (Wadud, 2004). It can be considered as Band-aid that is used in Conventional Medicine. However, Conventional Medicine has confined this dosage form only for haemostatic and as an antiseptic, but Unani system Unani System of Medicine is enriched with lot of formulations of lazooq (Khan, 2005; Kabeeruddin 2006) which can be developed and modified.
Shiyaf (Suppository)
In Unani Medicine, this preparation is used according to the site like ear, nose, urethra, rectum, vagina and wounds, etc, in the form of batti (cylindrical shape) and given different names viz; Hamool, Fateela and Farzaja. (Qureshi, 1995; Ghani, 2010) Conventional Medicine prepared this dosage form by using appropriate absorbent, absorption enhancers, diluents and lubricants. They commonly use theobroma, kokum, olive, glycerol and almond oil as base of suppository. Unani pharmaceutical laboratories can also prepare this dosage form with the help of these bases. It will be helpful in some emergency conditions like high grade fever, vomiting, constipation, local sepsis etc. It can also be useful in children and old patients particularly in cases of fever. (Tripathi, 2003).
Farzaja (Vaginal pessary)
Presently, this dosage form is rarely used in Unani System of Medicine (Said, 1997). Fatty bases, such as theobroma oil, or water soluble bases such as glycerol are used for the preparation of pessaries in Modern pharmaceutics. Bases should be formulated such that the pessaries melt at, or slightly below 37 °C (Lund, 2009).
Huqna (Enema)
Unani Medicine claims Jalinus introduced this dosage form. It is prepared with the help of ‘Roghan-e-Gul’ and ‘Roghan-e-Kunjud’ as the base of Huqna. Today, practitioners of Conventional Medicine also prefer ‘soap water enema’ that was previously preferred by Unani physicians. This may be prepared in a more scientific manner. This dosage form may be useful in inflammation of gastrointestinal mucosa, constipation and several emergencies and may also use for nutritive purpose in terminally ill patients (Ghani, 2010).
Khizab (Hair dye), Ghaza (Face powder), Ghalia (Perfumed powder), Norah (Hair remover), Sabeeg (Skin dye)
These are cosmetic dosage forms which are vital for beauty enhancements in today’s life. Razi has written a chapter of Alhawi fit tib volume VI and Kitab al-Mansuri on this topic (Razi, 1999; Razi, 1991). The author of Al Moalijat-e-Buqratiya has also given a lot of formulations for cosmetic disfigurements (Tibri, 1997). At present, only ‘Ghaza Husn-e-Afza’ prepared by Hamdard laboratories (Said, 1997) and ‘Kalonji Fairness Cream’ prepared by Mohammadia Dawakhana are being marketed. Unani soaps, shampoos, hair dyes and perfumed powder may be of wider use, if develop on a large scale due to its safety and efficacy.
Lakhlakha (Inhalers/ Aerosol)
Unani physicians used this dosage form in traditional manner. They advised the patients to take powder drug into a large opening of utensil and inhale. This dosage form was prepared at the time of administration of drug (Ghani, 2010). Now pharmaceutical laboratories prepare this dosage form as Aerosol which generally consists of solutions, emulsions, or suspensions of medicaments in a mixture of inert propellants which are held until required (Lund, 2009).
Saoot (Nasal drop), Qutoor (Eye & Ear drop)
Jalinus was the founder of Saoot. He prepared this dosage form for patients who felt inconvenience in taking medicine orally particularly in headache. Now it is used especially in nasal diseases. (Ghani, 2010) Hamdard pharmacopoeia has mentioned ‘Qutoor-e-Ramad’ and ‘Qutoor-e-Siyah’ (Said, 1997). However, these are not available in the market. A pre-clinical study has been done on conjunctivitis by Abdul-Lateef et al. (2010), who prepared an eye drop of Unani formulations and used in rabbits and result was found statistically significant (Abdul-Lateef et al, 2010).
Oily vehicle and several preservatives are found to stop cilial movements. In order to prevent deleterious effects on cilial action, the viscosity, tonicity and pH of nasal drops should be as near as possible to those of natural secretions; adjustment may be made by the addition of hypromellose, sodium chloride and buffers (Lund, 2009).
CONCLUSION
The compounding of Unani drugs is profoundly rooted in history. Ancient Unani physicians introduced varied dosage forms viz; Sufoof, Qurs, Shiyaf, Qutoor, Majun, Iitrifal, Laooq, Khameera and Sharbat etc. Arastu, Indrumakhas, Buqrat, Arab physicians and even Indian Hakims prepared and modified several dosage forms as per the necessities and public demand which kept the system going. In ancient period, all the dosage forms were accepted but now the period has highly been developed with social, cultural and even environmental state of affairs have been changed. Unani Medicine has been developed in several aspects but some areas are still unexplored. Nearly 90 dosage forms are documented in Unani pharmaceutical books, (Arzani, 1998; Said, 1997; Ibn Sina, 2006; Ghani, 2010; Kabeeruddin, 1935; Hafeez, 2005) but only 1/3rd is used in present era. Most of the dosage forms remain only an ornament of books because of varied reasons viz. time consuming preparations, not easy to carry, large bulk of doses, not so attractive, unsterilized method of handling, delayed action, high sugar content etc. These are the prime factors for not reaching Unani medicine to the public at large due to stagnation in drug dosage forms. In contrast to Unani Medicine, Conventional and Ayurvedic Medicines have changed drug dosage forms according to need of time and individuals. Those Unani drug dosage forms which are only an ornament of books e.g., Shiyaf, Saoot, Qutoor, Farzaja, Fateela, Lakhlakha, Khizab, Ghaza, Ghalia, Ubtana, Kohl, Zaroor, Nafookh, Qeruti, Lazooq, Norah and Shamoom etc., are to be explored and prepared in scientific manner. Those dosage forms which are already used but have certain draw backs they should also be modified viz; tablets may be prepared in varied forms, most of the semisolid dosage forms contained rich sugar contents may be transformed into tablets and capsule or they should be free from sugar because of high prevalence of diabetes mellitus. Most of the dosage forms may be prepared from extract to decrease bulk of dosage and increase efficacy of drugs.
To counter the advocacy of traditionalist physicians to maintain status quo in modification of drugs, stern efforts may be taken to conduct pre-clinical and clinical studies to compare the safety and efficacy of existing as well as modified drug dosage forms to arrive at the ideal dosage form. Unless and until the aforesaid measures are taken on priority basis, the survival of the system will remain a quagmire.
ACKNOWLEDGEMENT
The authors hereby acknowledge the librarian of Regional Research Institute of Unani Medicine, Chennai and Central Library of National Institute of Unani Medicine, Bangalore for providing necessary literature materials. The authors are also grateful to authors of those papers whose research papers were cited in this review.
CONFLICT OF INTEREST
None declared.
REFERENCES
Abdul-Lateef, Razique A, Sukul RR, Siddiqui N. Anti-inflammatory and Antihistaminic Study of a Unani Eye Drop Formulation. Ophthalmol Eye Disease. 2010; 2: 17-22.
Ali E. Qarabadeen-e-Ehsani. 2nd Edition. New Delhi: CCRUM, Dept. of AYUSH, Ministry of Health and Family Welfare, Govt. of India; 2006. p. 28-145.
Anonymous. The evolution of pharmacy Theme D, Level 1, the development of dosage forms; [https://www.rpharms.org] (Assessed on 12 October 2015)
Arzani A. Qarabadeen-e-Qadri. (Urdu translation). New Delhi: Ejaz Publishing House; 1998. p. 6, 9, 20, 22, 58, 60, 89.
Bari A. Jame al-Advia. Deoband: Faisal Publications; 2003. p. 104.
Chaudhary SS, Tariq M, Zaman R, Imtiyaz S. Solid dosage forms in Unani system of medicine. Journal of Pharmaceutical and Scientific Innovation. 2013; 2 (3): 17-22.
George AB. Great Movements in Pharmacy. Park, Davis & Company; 1965. p 10.
Ghani N. Khazain al-Advia. 1st Edition. New Delhi: Idarah Kitab al-Shifa; 2010. p. 108-124.
Hafeez A. Qarabadeen-e-Jadeed. New Delhi: Dept. of AYUSH, Ministry of Health and Family Welfare, Govt. of India; 2005. p. 25.
Ibn Abbas. Kamil al-Sana. Vol 2, Part II. (Urdu translation by Kanturi GH). CCRUM, Dept. of AYUSH, Ministry of Health and Family Welfare, Govt. of India; 2010. p. 479-492.
Ibn Sina. Al Qanoon fit Tib (Urdu translation by Kanturi GH) Vol. V. New Delhi: Dept. of AYUSH, Ministry of Health and Family Welfare, Govt. of India; 2006. p. 1-4.
Ibnul Quf, Kitabul Umdah Fil Jarahat (Urdu translation by CCRUM) Ministry of Health and Family welfare, Govt. of India, New Delhi, 1986. p. 102-107, 174-175, 234, 263, 268, 271-274, 292-293.
Jalaluddin. Qarabadeen-e-Jalali. 2nd Ed. New Delhi: CCRUM, Dept. of AYUSH, Ministry of Health and Family Welfare, Govt. of India; 2006. p. 2-134.
Jokhab S. Pharmacy History. [http://ksupc.com/download] [Assessed on 16 October 2015)
Kabeeruddin. Al-Qarabadeen. 2nd Edition. New Delhi: CCRUM, Dept. of AYUSH, Ministry of Health and Family Welfare, Govt, of India; 2006. p. 991-992.
Khan A. Qarabadeen-e-Azam va Akmal. (Urdu translation by CCRUM), New Delhi: Dept. of AYUSH, Ministry of Health and Family Welfare, Govt. of India; 2005. p. 2, 8, 11, 13, 16, 17, 20, 21, 23, 24, 31-32, 35, 36, 84, 88, 95, 317, 318, 321, 330, 331, 335, 362, 363, 378, 397, 405, 416, 419, 420, 421, 422, 430, 432, 433, 434.
Khan MS. Elaj al-Amraz. (Urdu translation by Kabeeruddin). New Delhi: Ejaz Publishing House; 2006. p. 912-944.
Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. 43rd Ed. Pune: Nirali Prakashan; 2009. p. 3.1.
Lateef A, Tafseer MB, Rauf A, Rehman S. Physico-chemical standardization of Laooq Sapistan Khayaar Shambari: A Pharmacopoeial Compound Unani Formulation. Pharmacophore. 2013; 4 (6) 268-274.
Lohar DR. Legal statuts of Ayurvedic, Siddha & Unani Medicines. Ghaziabad: Pharmacopoeial Laboratory for Indian Medicines. Dept. of AYUSH, Ministry of Health and Family Welfare, Govt. of India; YNM. p. 30.
Lund W. The Pharmaceutical Codex. 12th Ed. India: CBS Publishers & Distributors; 2009. p. 12, 24-27, 28-29, 128-132, 158, 175.
Meena R, Meena AK, Khan SA, Mageswari S. Evaluation of a Unani compound formulation-Majoon-e-Sandal. International Journal of Pharma Sciences and Research. 2010; 1(5): 238-242.
Pasupuleti A, Kumar S, Brahma CK. Formulation and evaluation of Oro-dispersible tablets of Deferasirox. International Journal of Research in Pharmaceutical and Nano Sciences. 2014; 3(6): 509- 516.
Patil J, Vishwajith V, Gopal V. Formulation development and evaluation of chewable tablets. Journal of Pharmaceutical and Scientific Innovation. 2012; 1 (3): 112-117.
Qureshi EH. Muqadma-e-Ilmul Advia. New Delhi: Ejaz Publishing House; 1995. p. 81-103.
Razi. Al Hawi fit Tib. Vol. VI. (Urdu translation by CCRUM). New Delhi: Dept. of AYUSH, Ministry of Health and Family Welfare, Govt. of India; 1999. p. 183-239.
Razi. Kitab al-Mansuri. 1st Ed. (Urdu translation by CCRUM). New Delhi: Dept. of AYUSH, Ministry of Health and Family Welfare, Govt. of India; 1991. p. 187-205.
Rehman Z. Kitab al-Murakkabat. Aligarh: Ajmal Khan Tibbiya College, AMU; 1991. p. 65, 99, 111, 114, 152, 158.
Said M. Hamdard Pharmacopoeia of Eastern Medicine. 2nd Ed. Delhi: Sri Satguru Publications; 1997. p. 65, 73-75, 77, 119, 152, 169,
Tibri A. Al Moalijat-e-Buqratiya. Vol. II. (Urdu translation by CCRUM). New Delhi: Dept. of AYUSH, Ministry of Health and Family Welfare, Govt. of India; 1997. p. 12-57, 107-110.
Tripathi KD. Essentials of Medical Pharmacology. 5th Edition. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2003. p. 8,176,488,715.
Vogel HG. Drug Discovery and Evaluation: Pharmacological Assays. 2ndEd.New York: Springer-Verlag Berlin Heidelberg; 2008. p. 2.
Wadud A. Ashrah al-Advia (Kulliyat-e-Advia). Burhan Pur: Printed by Mumtaz Screen Printer; 2004. p. 54-69.
World Health Organization. Review of World Pharmacopoeia, International Meeting of World Pharmacopoeias. Geneva: World Health Organization; 2013. P. 3-4. [http://www.who.org] (Accessed on 12 October 2015).
World Health Organization. WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues. Geneva: World Health Organization; 2007. P. 19. [http://apps.who.int org] (Accessed on 15 October 2015)









 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.