Vihari M, Siddikuzzaman, Berlin Grace VM *
Department of Biotechnology, Karunya University, Coimbatore-641 114, Tamil Nadu, India.
ORIGINAL RESEARCH ARTICLE
Volume 2, Issue 1, Jan-April 2014.
Article history
Received: 15 March 2014
Revised: 20 March 2014
Accepted: 20 April 2014
Early view: 28 April 2014
*Author for correspondence
E-mail: [email protected]
Mobile/ Tel.: +91 9894051175
Keywords:
ATRA
HeLa cells
Retinoid receptors
Cytotoxicity.
Background: All-trans retinoic acid (ATRA) is known to regulate cell growth, differentiation and apoptosis in human. However, its action on cancer cell proliferation is not clear whether it act through regulatory molecules at gene level or directly acts on the cells by inducing apoptosis and delaying cell growth. To analyze the effect of ATRA on the proliferation of cervical cancer cell line HeLa.
Materials and methods: The growth inhibitory effect was analyzed by MTT and Trypan blue assay. The morphology of HeLa cell lines after treatment with ATRA was also studied with the help of Haematoxyline and Eosin staining which showed an improved shape for the cells. This inhibition may be due to growth arrest or due to cell death by apoptosis and measured by TUNEL assay.
Results: The percentage inhibition of ATRA on HeLa cells were found to be in a dose dependent manner and a 78% of inhibition was observed for ATRA at 10-3mM concentration at 48 hours of treatment. The TUNEL assay result has shown higher percentage apoptotic cells (67 cells) per field for 10-3 mM ATRA treatment.
Conclusions: The study indicates that the ATRA has a very good growth inhibitory effect on HeLa cell line with an induction of apoptosis at significant level.
INTRODUCTION
Cervical cancer is the second most common cancer in women worldwide (Berlin-Grace et al., 2006; Zeng et al., 2002). In 2008, an estimated 5,30,000 cases of cervical cancer and 2,75,000 deaths from the disease was reported (Berlin-Grace et al., 2006; Zeng et al., 2002). An estimated 12,800 new cases of cervical cancer were diagnosed in the United States and in 1999, 4800 patients have died of this disease. In India, the highest cervical cancer frequency with 134,000 cases and 73,000 deaths were reported in 2008 (Arbyn et al., 2011). The highest rate of incidence is observed in Guinea, with 6.5% of women developing cervical cancer before 75 years of age (Arbyn et al., 2011). Therefore, it is necessary to search a novel approach to combat cervical cancer. We have reported a direct association of reduced serum ATRA level with different stages of cervical cancer in patients (Berlin-Grace et al., 2006).
Till now there is no curable drug discovered for cervical cancer (Huang et al., 1998). Retinoic acid, being a metabolite of vitamin A plays a vital role in prevention of cancer development. It is a lipophilic molecule, that effects gene transcription by acting as a transcription factor and modulates a variety of biological processes like cell proliferation, differentiation and apoptosis (Malcolm and Matthew, 2003). The most active form of vitamin A (Retinoids) is all-trans retinoic acid (ATRA), derived mostly from animal sources such as liver, egg, milk as retinol/retinyl ester or from plant sources as carotinoid precursor. It plays a multirole in body functions, it is vital for immune system, maintenance of growth and development of tissues. The deficiency of vitamin A can lead to risk of cancer development (Basu et al., 2003). The effects of retinoids are mediated through two classes of receptors-RAR and RXR. Both of them have α, β and γ subclasses with various isoforms (Malcolm and Matthew, 2003). An in vitro growth inhibition study of ATRA on thyroid tumor cell line has reported that both apoptosis and decrease in DNA synthesis in terms of delay in cell cycle progression are the mechanism of growth inhibition (Elisei et al., 2005). A significant number of cancers are often failed to respond to the conventional chemotherapy, radiotherapy and surgery with high percent of recurrence. ATRA is being explored as a potent drug for cancer therapies. ATRA is clinically proven to be an efficient and sole therapeutic drug for acute promyelocytic leukemia (APL) and is a good anti-viral agent too (Gianni et al., 2000). The cervical cancer cell line HeLa is a human papilloma virus (HPV) infected cancer cell line. Hence, the ATRA may have a therapeutic effect on HeLa cell line. The ideal therapeutic target for retinoids is cancers of epithelial tissue origin which include cervical cancer because of their growth and
differentiation are modulated by retinoids (Lippman et al., 1987, 1987a).
The purpose of this study is to find out whether ATRA has direct action on inhibition of cancer cell proliferation, cytotoxicity and induction of apoptosis in HeLa cells in vitro.
MATERIAL AND METHODS
Cell culture
Human cervical cancer cell line HeLa was purchased from National Center for Cell Sciences (NCCS) Pune, India. The HeLa cells were incubated in CO2 incubator with 37ºC atmosphere and 5% CO2 until confluent. The cells were harvested in RPMI-1640 and supplemented with penicillin, streptomycin, and 10% fetal calf serum (FCS). When the HeLa cells were halted to produce 70-80% confluent cultures, the cells were isolated by using trypsin-EDTA and adjusted to 1X106 cells/mL in phosphate-buffered saline (PBS, pH 7.4).
Chemicals
ATRA was purchased from Sigma Chem. Co. (St. Louis, MO). All other chemicals used were of analytical grade.
Study on the growth inhibition effect of ATRA on HeLa cells by MTT assay
MTT (3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay was performed after 24 and 48 hours drug treatment. HeLa cells (1×104 cells) were harvested in 96-well plates. After 24 hours of cell seeding, different concentrations of ATRA (10-3, 10-6, 10-9 mM) were added to each well. Before 4th hour of the completion of incubation at next 24 hrs and 48 hrs, 20µl of MTT (Sigma Aldrich) (5 mg/mL) was added to each well including the control wells (without ATRA) (Cole, 1986; Campling et al., 1991). Well plates were centrifuged at 2000 rpm for 10 min; medium was then removed, and 100 µL of DMSO was added to each well. After thorough mixing with a mechanical plate mixer, absorbance of the wells was read at a wavelength of 570 nm. Percentage growth inhibition was expressed over the control. All assays were taken in 6 replicates for statistical analysis.
Study on the cytotoxic effect of ATRA on HeLa cells by Trypan blue dye exclusion method
Short term cell death study by trypan blue exclusion method is a very simple method which was carried out within a short period of 3 hours. HeLa cells (1×104 cells/wells) were incubated with various concentrations (10-3, 10-6, 10-9 mM) of ATRA in a final volume of 1mL for 3 hours at 370C. After incubation, the cytotoxicity was determined by counting the dead cells (Talwar, 1974).
Study on the cytopathologic effect of ATRA on HeLa cell morphology by Hematoxylin and Eosin (H&E) staining.
HeLa cells were grown on cover slips with 70-80% confluence, followed by incubation with different concentrations of ATRA (10-3, 10-6, 10-9 mM) for 24 hrs. Control (untreated) and the treated cells were washed with cold PBS (phosphate-buffered saline: 137 mM NaCl, 2.7 mM KCl, 10mM Na2HPO4, 1.76m MKH2PO4, pH7.4) and then the cells were fixed with 4% paraformaldehyde in PBS for 20 mins at room temperature. The cells were washed with PBS for 3 times and treated with 0.2% Triton X-100 in PBS for 5 minutes. The cells were again washed 3 times with cold PBS followed by staining with H&E at 37 °C for 1 hour (Ming et al., 2012). The stained cells were washed 3 times with cold PBS, mounted on a microscopic slide with glycerol, and the morphology of the HeLa cells were visualized under light microscope. The cytopathologic changes were verified by a chief pathologist.
Study on the effect of ATRA on induction of apoptosis in HeLa cells by TUNEL assay
For detection of apoptosis, in situ DNA fragmentation in HeLa cells was performed by using Dead End Colorimetric TUNEL system (Promega, Madison, WI) as per the recommendation of the manufacturer. Briefly, cells were fixed in poly-L-lysine coated slides and then washed with PBS followed by fixation with 4% paraformaldehyde and permeabilized with proteinase K solution (10 mg/ml). Cells were incubated with TdT at 37°C for 60 min. The activity of TdT was terminated by addition of 2X saline sodium citrate. The endogenous peroxidase was blocked by 0.3% H2O2. After mounting with 80% glycerol the slides were observed under a light microscope (Gavrieli et al., 1992). For the quantification of TUNEhttp://biomedjournal.com/wp-admin/post-new.php#L expression (i.e. TUNEL score), positive cells (intense brown) were counted in six random fields (Suresh et al., 2001). Cells that had granular brown pigment in the nucleoplasm were scored as apoptosis positive.
Statistical analysis
All data were expressed as mean ± S.D. The statistical analysis was done using one-way analysis of variance (ANOVA) followed by a Dunnett’s test (using Graphpad InStat version 3.00 for Windows XP; GraphPad Software, Inc., La Jolla, CA). P-value ≤ 0.05 was considered significant.
RESULTS
Growth inhibitory effect of ATRA on HeLa cell line by MTT assay
The results obtained in MTT assay reveals that the growth inhibitory or anti-proliferative effect of ATRA increase in time and dose dependent manner as shown in Fig. 1.
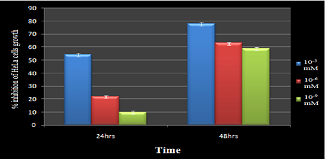 |
Figure 1. Growth inhibition activity of ATRA toward HeLa cells by MTT assay: Cytotoxic effect of ATRA towards HeLa cell line was determined by the trypan blue exclusion method and the percentage of viable cells, dead cells were Click here to view full image |
This is expressed in percentage of inhibition on cell growth in comparison with control growth inhibition. The highest percent (78%) is observed for 10-3 mM ATRA treatment at 48th hour of treatment.
Level of HeLa cell death by Trypan blue assay
The death rate of HeLa cells increased (16 to 62%) with increase in concentration of ATRA treatment (10-9 to 10-3) as shown in Fig. 2.
Figure 2. In vitro cytotoxicity effect of ATRA towards HeLa cell line by Trypan blue exclusion method: Percentage of growth inhibitory caused by ATRA is shown. Values shown are expressed as mean ± S.D. (n = 6 determinations/dose)..
 |
Figure 2 In vitro cytotoxicity effect of ATRA towards HeLa cell line by Trypan blue exclusion method: Percentage of growth inhibitory caused by ATRA is shown. Values shown are expressed as mean ± S.D. (n = 6 determinations/dose). |
Effect of ATRA on the HeLa cell morphology by H & E staining
The morphological changes of HeLa cells were observed by light microscope. On light microscopic examination of HeLa cells, the nuclei were clearly visible. The shape of the membrane, however, differed according to the treatments. In untreated cells, the membranes were nearly circular, but changed into irregular polygons following ATRA treatment. After treatment with ATRA, HeLa cells showed physical changes: the cells became oval, and the surface became extremely rough. Because of the nuclei of the treated cells had exploded, as indicated by the large area of the bright region.
Effect of ATRA on induction of apoptosis by TUNEL assay
The TUNEL signal, as an apoptosis marker, appeared as dark brown nuclei as shown in Fig. 3 and the TUNEL score in terms of number of positive cells are given in Fig. 4. More number of apoptotic positive cells was observed with increase in ATRA concentration. Treatment with 10-3 mM has shown 67 ± 3.0 TUNEL positive cells and that of for 10-6 mM as 44 ± 2.0, and 10-9 mM as 30 ± 3.0. The control has shown only 5 ± 1.0 apoptotic positive cells per field.
DISCUSSION
To stop the progression of preneoplas¬tic lesions, chemoprevention is an important strategy in cancer therapy (Sun and Lotan, 2002). The natural derivatives of vitamin A are ATRA, 9-cis retinoic acid (9-cRA) and 13-cis retinoic acid (13-cRA), currently being used in the treatment of cancer. Retinoids are very effective in suppressing the development of tumor in many animal models of carcinogenesis and are being evaluated for treatment and prevention of cancers (Swift et al., 2006; Tang and Gudas, 2011).
Figure 3. Apoptosis positive cells in the ATRA treatment on HeLa cells: (a) HeLa cells treated with DMSO after 24 hrs as control with only five number of TUNEL positive cells which might be the normal level of apoptosis. (b) HeLa cells treated with 10-9 mM concentration ATRA for 24 hrs shows minimum number of positive apoptotic cells, but more than the control. (c) HeLa cells treated with 10-6 mM concentration ATRA for 24 hours shows few numbers of cells with apoptotic positive signal. (d) HeLa cells treated with ATRA with concentration 10-3 mM for 24 hours shows maximum number of positive apoptotic cells when compared to other concentrations and control.
 |
Figure 3 Apoptosis positive cells in the ATRA treatment on HeLa cells: (a) HeLa cells treated with DMSO after 24 hrs as control with only five number of TUNEL positive cells which might be the normal level of apoptosis. (b) HeLa cells treated with 10-9 mM concentration ATRA for 24 hrs shows minimum number of positive apoptotic cells, but more than the control. (c) HeLa cells treated with 10-6 mM concentration ATRA for 24 hours shows few numbers of cells with apoptotic positive signal. (d) HeLa cells treated with ATRA with concentration 10-3 mM for 24 hours shows maximum number of positive apoptotic cells when compared to other concentrations and control. |
Retinoids are known for regulation of gene expression, which leads to inhibition of cellular proliferation, induction of differentiation and apoptosis in a variety of cancer cells (Crowe et al., 2003; Gumireddy et al., 2003; Zeng et al., 2002). Various studies and clinical trials have revealed the anti-leukemic effect of ATRA in patients with acute promyelocytic leukemia (Siddikuzzaman and Berlin-Grace, 2012). However, the in vitro studies on the effects of ATRA on cancer cell lines to show its direct action on cancer cells are limited. As vitamin A plays a vital role in reproduction, its metabolite retinoids may play a major role in regulating the epithelial cell growth and differentiation in the uterine cervix. The mode of action of retinoids in cervical cancer is still unclear. The results of the present study may provide interesting new insights for the understanding of the mechanisms of action of anti-cancer effect of ATRA on HeLa cells. Retinoid signaling is often com¬promised early in carcinogenesis. Through the retinoids the receptors (RARs and RXRs), are ligand-dependent transcription factors, they either induce or repress the transcription of tar¬get genes.
 |
Figure 4 Figure 4. Number of apoptosis positive cells HeLa cells upon ATRA treatment: Apoptotic positive cells per field were observed. Values were expressed in mean ± S.D. (n=6). The significant level was determined between control vs. 10-3 mM ATRA treatment (**P ≤ 0.01). Control vs. 10-6 mM ATRA treatment (**P ≤ 0.01). Control vs. 10-9 mM ATRA treatment (**P ≤ 0.01). |
We observed a 78% growth inhibition for 10-3 mM ATRA at 48 hours on HeLa cells by MTT assay which may be due to delaying the cell cycle as well as induced apoptotic death of cells. A delay of cell cycle progression was already demonstrated for ATRA by a previous study on NPA cell line (Elisei et al., 2005). The retinoid suppression of growth does not involve the death of tumor cells but rather arrested during the G1 stage of the cell cycle (Wu et al., 1997). Further, we found that the ATRA could also influence the cytopathology of HeLa cells. The characteristic round morphology of cancer cells also started changing to oval shape and surface became very rough with reduced cytoplasm as observed in the H & E stain after ATRA treatment.
The significant in vitro inhibition on cell proliferation observed in our study may also be due to the death of HeLa cells after growth. Hence, we performed the cytotoxicity study by trypan blue assay to check for dead cells. We have observed a significant number of dead cells (62 ± 3 cells for 10-3 mM) also in ATRA treatment which was also dose dependent action. Then we performed TUNEL assay to find out whether the dead cells were by increased level of apoptosis induced by ATRA in HeLa cell. As we expected, we found 67 ± 3 apoptotic positive cells per field in 10-3 mM ATRA treatment. In this study, ATRA was found to have cytotoxic effect on Hela cells at a dose dependent manner i.e. 10-3 mM showed more toxicity effect as compared to 10-9 mM treatment. The HeLa Cells undergoing apoptosis have shown a characteristic morphology: Cell shrinkage and rounding, cytoplasm appeared dense, and the organelles appeared tightly packed. The cell membrane showed irregular buds and the cell broke apart into several vesicles called apoptotic bodies. It was already reported that high concentration and long exposure time of ATRA was required to induce cell apoptosis (Hsu et al., 1998).
The induction of apoptosis was already reported as an effective therapeutic approach with many chemotherapeutic agents showing their antitumor effects in cancer cells (Kamesaki, 1998; Ricci and Zong, 2006). The TUNEL assay in our study has thus confirmed that the cell death observed in trypan blue assay could be induced due to the apoptosis. TUNEL is a highly sensitive procedure designed to detect free 3’OH single-strand DNA breaks produced by DNA fragmentation, typically localized to cell death (Siddikuzzaman and Berlin-Grace, 2012). The induction of apoptosis by a polysaccharide treatment on HeLa cells was already reported (Xiao et al., 2007). In our study, higher concentration of ATRA induced higher number of apoptosis positive cells, whereas 10-9 mM ATRA showed less apoptosis positive cells. So, it is clear that the 10-3 mM ATRA can enhance apoptosis significantly in HeLa cells when compared with untreated control cell line. We found good association in the levels of inhibition of HeLa cell line growth or proliferation, cell death (cytotoxicity) and apoptosis positive cells induced by ATRA treatment at 10-3 mM concentration as shown in Fig. 1, 2 and 4. This in vitro study indicates a significant action of ATRA directly on stopping HeLa cell line proliferation. Studies using topically applied Vitamin A to the cervix have resulted in up to 50% complete reversal of cervical dysplasia in phase-II and III clinical trials (Lippman et al., 1993). This suggests that the application of ATRA directly may have better result. In our previous studies ATRA has been reported to exhibit anti-cancer activity against in vivo lung cancer in mice models (Siddikuzzaman et al., 2011; Siddikuzzaman and Berlin-Grace, 2012).
Further study is required to find out to what extent the ATRA is inhibiting growth of the cancer cells or enhancing the apoptosis for cancer cells death in vitro for better understanding of its mechanism of action.
CONCLUSION
The present study indicates that the ATRA at 10-3 mM concentration effectively inhibit the HeLa cell growth in vitro and significantly induce the process of apoptosis. Furthermore, the ATRA treated HeLa cells showed morphological changes which were an indication of anti-cancer effect of ATRA. Therefore, this helps for the understanding of how retinoids work as chemopreventive or chemotherapeutic agents in cervical cancer cells. So, we can propose that ATRA can be a potent chemotherapeutic agent against several diseases including cancer which are ATRA sensitive. However, in solid cancer patients, the therapeutic outcome was not as efficient as it works out for APL, which may be due to many other factors such as reach ability of ATRA in active form to the target site, concentration and so on. Further study is warranted to analyze the mechanisms in detail.
ACKNOWLEDGMENT
The authors would like to acknowledge the financial support and research facilities rendered by the Karunya University through Karunya Short-Term Research Project Grant (KSTPG) for this study.
CONFLICT OF INTEREST
None declared.
REFERENCES
Arbyn M, Castellsague X, De Sanjose S, Bruni L, Saraiya M, Bray F, Ferlay J. Worldwide burden of cervical cancer in 2008. Annals of Oncology. 22, 2675-2686, 2011.
Basu S, Sengupta B, Paladhi PK. Single megadose vitamin A supplementation of Indian mothers and morbidity in breastfed young infants. Postgrad Med J. 79, 397-402, 2003.
Berlin-Grace VM, Niranjali Devaraj S, Radhakrishnan PM, Devaraj H. HPV-induced carcinogenesis of the uterine cervix is associated with reduced serum ATRA level. Gynecol Oncol. 103, 113-119, 2006.
Campling BG, Pym J, Baker HM, Cole SP, Lam YM. Chemosensitivity testing of small cell lung cancer using the MTT assay. British J Cancer. 63, 75-83, 1991.
Cole SP. Rapid chemosensitivity testing of human lung tumor cells using MTT assay. Cancer Chemother Pharmacol. 17, 259-63, 1986.
Crowe DL, Kim R, Chandraratna RA. Retinoic acid differentially regulates cancer cell proliferation via dose-dependent modulation of the mitogen-activated protein kinase pathway. Mol Cancer Res. 1, 532-540, 2003.
Elisei R, Vivaldi A, Agate L, Ciampi R, Molinaro E, Piampiani P, Romei C, Faviana P, Basolo F, Miccoli P, Capodanno A, Collecchi P, Pacini F, Pinchera A. All-trans-retinoic acid treatment inhibits the growth of retinoic acid receptor beta messenger ribonucleic acid expressing thyroid cancer cell lines but does not re-induce the expression of thyroid-specific genes. J Clin Endocrinol Metab. 90, 2403-2411, 2005.
Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 119, 493–501, 1992.
Gianni M, Ponzanelli I, Mologni L, Reichert U, Rambaldi A, Terao M, Garattini E. Retinoid-dependent growth inhibition, differentiation and apoptosis in acute promyelocytic leukemia cells (APL). Expression and activation of caspase. Cell Death Differ. 7, 447-460, 2000.
Gumireddy K, Sutton LN. Phillips PC, Reddy CD. All-trans-retinoic acid-induced apoptosis in human medulloblastoma: Activation of caspase-3/poly (ADPribose) polymerase 1 pathway. Clin Cancer Res. 9, 405-409, 2003.
Hsu SL, Wu WS, Tyan YS, Chou CK. Retinoic acid-induced apoptosis is prevented by serum albumin and enhanced by lipiodol in human hepatoma Hep3B cells. Cancer Letters. 129, 205–14, 1998.
Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, Gu LJ, Wang ZY. Use of All-trans retinoic acid in the treatment of acute promyelocytic leukemia (APL). Blood. 72, 567-572, 1988.
Kamesaki H. Mechanisms involved in chemotherapy-induced apoptosis and their implications in cancer chemotherapy. Int J Hematol. 68, 29-43, 1998.
Lippman SM, Batsakis JG, Toth BB, Weber RS, Lee JJ, Martin JW, Hays GL, Goepfert H, Hong WK. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. N Engl J Med. 328, 15–20, 1993.
Lippman SM, Kessler JF, Meyskens FLJr. Retinoids as preventive and therapeutic anticancer agents (Part I). Cancer Treat Rep. 71, 391–405, 1987.
Lippman SM, Kessler JF, Meyskens FLJr. Retinoids as preventive and therapeutic anticancer agents (Part II). Cancer Treat Rep. 71, 493–514, 1987a.
Malcolm M, Matthew H. Retinoic acid, a regeneration-inducing molecule. Dev Dyn. 226, 237–244, 2003.
Ming CZ, Wen XH, En JG, Lin L, Ying Z, Lei D, Ren SW, Bo W, Mei LW. Synthesis, characterization, and cytotoxicity in vitro of the complex [Mn (Hptc) (phen) (OH)]n. Life Sci. 90, 519–524, 2012.
Ricci MS, Zong WX. Chemotherapeutic approaches for targeting cell death pathways. The Oncologist. 11, 342-357, 2006.
Siddikuzzaman, Berlin-Grace VM. Inhibition of metastatic lung cancer in C57BL/6 mice by liposome encapsulated all-trans retinoic acid (ATRA). Int Immunopharmacol. 14, 570–579, 2012.
Siddikuzzaman, Guruvayoorappan C, Berlin-Grace VM. All-trans retinoic acid and cancer. Immunopharmacol Immunotoxicol. 33, 241-249, 2011.
Sun SY, Lotan R. Retinoids and their receptors in cancer develop¬ment and chemoprevention. Crit Rev Oncol Hematol. 41, 41–55, 2002.
Suresh G, Stan L, Michael AG, Raffit H, Darrell BK, Ivey RT, Doris MB. Effects of retinoids on cancerous phenotype and apoptosis in organotypic cultures of ovarian carcinoma. J Natl Cancer Inst. 93, 516-525, 2001.
Swift ME, Wallden B, Wayner E, Swisshelm T. Truncated RAR beta isoform enhances proliferation and retinoid resistance. J Cell Physiol. 209, 718–725, 2006.
Talwar GP. Handbook of practical immunology. New Delhi. National Book Trust. pp. 336-339, 1974.
Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Am J Pathol. 6, 345–364, 2011.
Wu ZY, Gota S, Jollet F, Pollak M, Gautier SM, Natoli CR. Characterization of iron oxides by x-ray absorption at the oxygen K edge using a full multiple-scattering approach. Phys Rev. B 55, 2570–2577, 1997.
Xiao JX, Huang GQ, Zhu CP, Ren DD, Zhang SH. Morphological study on apoptosis hela cells induced by soyasaponins. Toxicol In Vitro. 21, 820-826, 2007.
Zeng M, Kumar A, Meng G, Gao Q, Dimri G, Wazer D, Band H, Band V. Human papilloma virus 16 E6 oncoprotein inhibits retinoic X-receptor-mediated transactivation by targeting human ADA3 coactivator. J Biol Chem. 277, 45611–45618, 2002.









 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.