Babar Ali1, 2*, Showkat R Mir2, Mohammad Ali2, Saiba Ali3
1College of Pharmacy and Dentistry, Buraydah Colleges, Buraydah, Al-Qassim, PO-31717, Kingdom of Saudia Arabia.
2Department of Pharmacognosy and Phytochemistry, Faculty of Pharmacy, Jamia Hamdard (Hamdard University), New Delhi-110062, India.
3Siddhartha Institute of Pharmacy, Dehra Dun, 248001, Uttarakhand, India.
SHORT COMMUNICATION
Volume 2, Issue 3, Page 158-161, September-December 2014.
Article history
Received: 1 October 2014
Revised: 10 October 2014
Accepted: 14 October 2014
Early view: 17 October 2014
*Author for correspondence
E-mail: [email protected]
Piper longum Linn. belongs to piperaceae family, is a renowned plant in the field of Ayurveda. Adulteration and substitution may affect the quality of Ayurvedic formulation. Standardization of Ayurvedic preparation is essential for further therapeutic results and for global acceptance. Hence, chromatographic fingerprint profiles were carried out for establishing the standards. TLC studies for methanolic extracts of the fruits of Piper longum were carried out in two different solvent systems, Toluene: Ethyl acetate: Diethyl ether (10:3:1) and Benzene: Ethyl acetate: Diethyl ether (6:3:1). The developed plates were dried in air, visualized in UV at wavelengths 254 nm and 366 nm and photographed. Results provide valuable clue regarding their polarity and selection of the solvents for separation of phytochemicals. Fingerprinting of methanolic extract of fruits of revealed the presence of various phytochemicals in UV at 254 nm and 366 nm. Fingerprint profile is quite helpful in setting up of standards and thus to keep a check on intentional/unintentional adulteration. TLC offers major advantages over other conventional chromatographic techniques such as unsurpassed flexibility (especially stationary and mobile phase), choice of detection wavelength, user friendly, rapid and cost effective/economical.
Keywords: Piper longum Linn., Ayurvedic formulation, Standardization, Thin layer chromatography.
INTRODUCTION
Piper longum Linn. (Piperaceae) commonly known as long pepper, sometimes called Javanese, Indian or Indonesian long pepper, is a flowering vine cultivated for its fruits which is usually dried and used as a spice and seasoning. The word pepper itself is derived from the Sanskrit word for long pepper, Pippali. The fruits contain the alkaloid piperine, which contributes to their pungency (Kirtikar and Basu, 2000). The plant grows all over India, in evergreen forests and is cultivated in Assam, Tamilnadu and Andhra Pradesh. The herb also grows wild in Malaysia, Singapore, Bhutan and Myanmar (Anonymous, 2003). The dried fruit spikes are extensively used for flavouring a variety of foods. They are considered to have stimulant, carminative, laxative and stomachic properties. The berries are also given with honey to treat asthma, coughs and sore throats. The root is a stimulant and is also used to cure gout, rheumatism and lumbago. The whole plant is considered by tribal people in India to be useful in splenic disorders, cholera, dysentery, asthma, cough and bronchitis (Satyavati et al., 1987). The fruits are aromatic, stimulant, carminative and prescribed to relieve constipation, gonorrhea, paralysis of the tongue, diarrhea, cholera, chronic malaria and viral hepatitis. P. longum is most commonly ingested to prevent respiratory infections such as stomachache, bronchitis, diseases of the spleen, cough, tumors, and asthma (Sharma et al., 2005). When applied topically, it soothes and reduces muscular pains and inflammation (Pei, 1983). Since it is so widely used in cooking and traditional medicine, it is generally assumed to be safe in moderate doses (Inder et al., 2007; Govindarajan, 1977).
It has been observed that the plant is used in many Ayurvedic formulations. Trikatu is a part of Ayurvedic formulation, containing P. longum as one of the major ingredient, enhances bioavailability of the various drugs when administered along with them (Anonymous, 1979; Antarkar and Vaidya, 1983). Quality of formulation depends on availability of genuine raw material. Popularity and demand increases the chance of adulteration and substitution. Like many other Ayurvedic herbs even Pippalimula is facing problems of non availability in market (KavirarShastri, 2005). Standardization of Ayurvedic medicine is essential for further therapeutic results and for global acceptance (Ujjaliya et al., 2012). Hence chromatographic fingerprint profiles were carried out for establishing the standards. Thin layer chromatography has progressed so rapidly that in many fields it has surpassed paper chromatography, chiefly because it is usually quicker and gives better separations. The method is used for separation of the components present in the mixture both qualitatively as well as quantitatively (Ali, 1998; Mukharjee, 2002; Anonymous, 1998). The development of such fingerprint for the fruits of Piper longum Linn is useful in differentiating the species from the adulterant and also acts as biomarker for this plant in the Pharmaceutical industry.
MATERIALS AND METHODS
Collection of materials
Fruits of Piper longum Linn. were procured from Green Earth Products Pvt. Ltd., Delhi, India and identified by Dr. H.B. Singh, Head, Raw Materials Herbarium and Museum, National Institute of Science Communication and Information Resources (NISCAIR), New Delhi. A voucher specimen with reference number NISCAIR/RHMD/1147/179/02 has been retained in the herbarium for future reference.
Preparation of plant material
The powdered drug material (100 g) was extracted with 250 ml of methanol in a Soxhlet apparatus for 8 hours and filtered. The extract was concentrated under reduced pressure and used for TLC fingerprinting.
Steps involved in TLC
The methanolic extract of crude drug powder was subjected to TLC to find out the number of compounds present in it. The different steps followed in the development of thin layer chromatographic profile of plant extract are as follows (Mukherjee, 2002):
(a) Preparation of test sample: The crude drug powder 10 g was extracted separately with methanol by heating on a water bath. The extract was filtered and concentrated.
(b) Preparation of plates: The absorbent silica gel G, about 25 g was taken in a glass mortar and about 35 ml of distilled water was added to it. The mixture was stirred with a glass rod until it became homogenous. The mixture was then allowed to swell for about 15 min. then additional 15 ml distilled water was added to it with stirring. The suspension was then transferred to a 150 ml flask fitted with a plastic stopper and was shaken vigorously for about 2 min. The suspension was then spread immediately on TLC plate (5 × 10 cm & 10 × 10 cm) to form a thin layer of the slurry.
(c) Drying and storage of plates: The freshly coated plates were then air dried until the transparence of the layer had disappeared. The plates were then stacked in a drying rack and were heated in an oven for 30 min at 110 °C. The activated plates were kept in desiccators till required for further use.
(d) Application of sample: Glass capillaries were used for applying test sample on TLC plates by keeping a minimum distance of 1 cm between the two adjacent spots. The spots of the sample were marked on the top of the plates to know their identity.
(e) Chromatographic chamber, condition of saturation and development of TLC plates: Chromatographic rectangular glass chamber (16.5 × 29.5 cm) was used to develop the plates. To avoid insufficient chamber saturation and the undesirable age effect, a smooth sheet of filter paper approximately of 15 × 40 cm size was placed in the chromatographic chamber in U-shape in developing solvent. After being moistened, the paper was pressed against the walls other chamber, so as to adhere it to the walls. The chamber was allowed to saturate for 1.5 h before use. The experiment was carried out at room temperature in diffused daylight.
(f) Development of solvent system: A number of developing solvent systems were tried, for methanolic extract by changing their concentration and combination to achieve satisfactory resolution.
(g) Development of chromatogram: The spotted plates were kept in chromatographic chamber containing the appropriate solvent system. The chamber was covered with greased plate. The solvent system was allowed to run up to ¾ of the height of the TLC plate. The plates were taken out and air-dried after marking the solvent front.
(h) Detection of spots: The air-dried plates were examined in daylight and in UV light (254 nm and 366 nm) for location of spots. The observations were recorded in Table 1.
TLC fingerprinting studies
Solvent systems were developed for establishing the TLC patterns for the methanolic extracts of the selected drugs. Various visualization techniques were used to come up with the best TLC fingerprint, like UV 254, UV 366, iodinisation and spray reagents like anisaldehyde, ninhydrin, aniline phthalate, Folin’s reagent, vanillin and sulphuric acid were also tried. The developed plates were dried in air, visualized in UV at wavelengths 254 nm and 366 nm and photographed (Wagner and Bladt, 1996).
RESULTS
Thin layer chromatography result confirmed the presence of different bioactive compounds (Figure 1 and 2). The result and observations were summarized in Table 1. TLC profiling of methanolic extract of P. longum fruits inToluene: Ethyl acetate: Diethyl ether (10:3:1) and Benzene: Ethyl acetate: Diethyl ether (6:3:1) confirms the presence of diverse biomolecule in the plant.
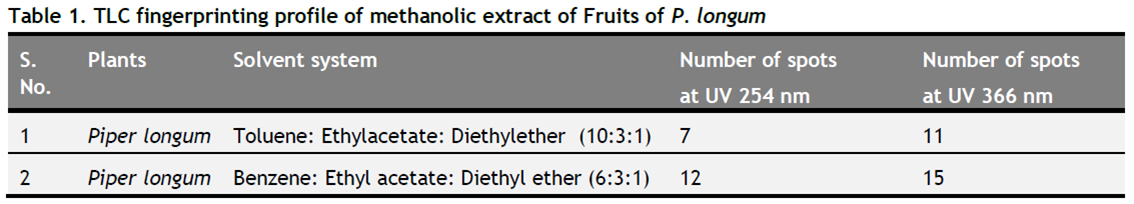 |
Table1. TLC fingerprinting profile of methanolic extract of Fruits of P. longum. Click here to view full image |
 |
Figure 1. Chromatogram of methanolic extract of Piper longum (fruits extract) in Toluene: Ethyl acetate: Diethyl ether (10:3:1), UV 254 nm and 366 nm. (1A) Methanolic extract (254 nm); (1B) Methanolic extract (366 nm). Click here to view full image |
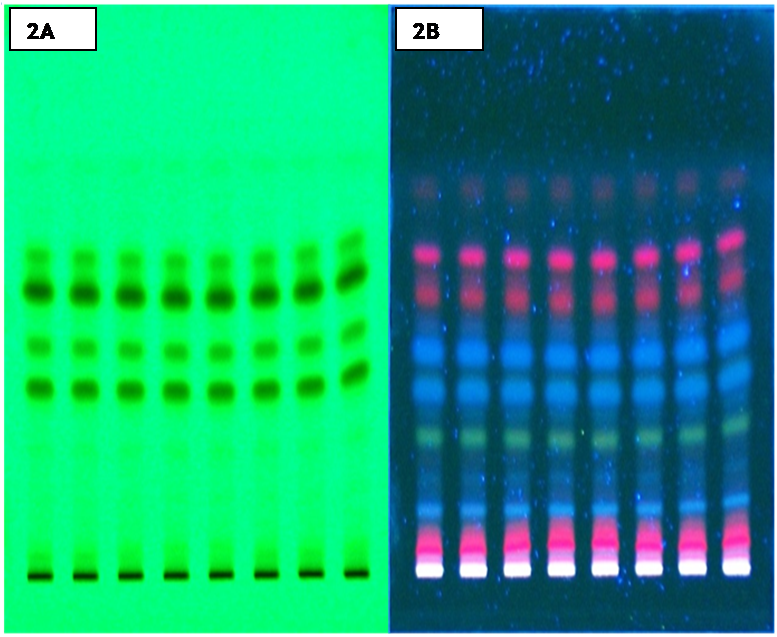 |
Figure 2. Chromatogram of methanolic extract of Piper longum (fruits extract) in Benzene: Ethyl acetate: Diethyl ether (6:3:1), UV 254 nm and 366 nm. (2A) Methanolic extract (254 nm); (2B) Methanolic extract (366 nm). Click here to view full image |
DISCUSSION
As herbal preparations have chemical complexity it is very difficult to identify all of their constituents. TLC analysis provides an idea about polarity of various chemical constituents, in a way such that compound showing high Rf value in less polar solvent system have low polarity and with less Rf value have high polarity (Dutta, 2013). In the present study the fingerprint profile of methanolic extract under UV light at 254 nm showed 7 and 12 spots where as at 366 nm showed 11 and 15 spots respectively for both solvent systems. Thus TLC chromatogram was observed best at 366 nm. The corresponding Rf values of various chemical constituents for Toluene: Ethyl acetate: Diethyl ether at 366 nm were 0.11 (pink), 0.14 (blue), 0.19 (light blue), 0.22 (light green), 0.25 (light blue), 0.28 (blue), 0.35 (blue), 0.41 (light pink), 0.56 (light pink), 0.82 (dark pink), 0.94 (light blue) and for Benzene: Ethyl acetate: Diethyl ether were 0.12 (light pink), 0.16 (pink), 0.25 (light pink), 0.36 (blue), 0.40 (light blue), 0.45 (light green), 0.50 (light blue), 0.55 (blue), 0,58 (blue), 0.61 (light blue), 0.72 (pink), 0.80 (light pink), 0.82 (pink), 0.85 (light pink), 0.94 (light pink) recorded respectively.
CONCLUSION
Fingerprint profile is quite helpful in setting up of standards and thus to keep a check on intentional/unintentional adulteration. Above chromatographic fingerprint analysis have significance in this regard. TLC showed presence of different compounds in both wavelengths. These constituents can be further used for development of different drug in future. Further detailed analysis of percentage of chemical constituents and clinical studies of long pepper should be done to evaluate comparative therapeutic efficacy.
CONFLICT OF INTEREST
None declared.
REFERENCES
Ali M. High performance thin layer chromatography for herbal and pharmaceutical product analysis. Indian J Pharm Ed and Res. 32, 15-19, 1998.
Anonymous. Handbook of Domestic Medicine and Common Ayurvedic Remedies, Central Council for Research in Indian Medicine and Homeopathy: New Delhi, India, 1979.
Anonymous. Quality control methods for medicinal plant materials. WHO, Geneva, 1998.
Anonymous. The Wealth of India, a dictionary of Indian raw materials and industrial products, raw materials. 1st supplementary series, J-Q4, CSIR, Publications and Information Directorate (PID): New Delhi, India, 2003.
Antarkar DS, Vaidya AB. Therapeutic approach to malaria in Ayurveda, Symposium on recent advances in protozoan diseases. Editors: Subrahmanyam D, Radhakrishna V, Hindustan Ciba - Geigy Research Centre, Goregaon, Bombay, India, pp. 96-101, 1983.
Dutta J. Phytochemicals analysis and TLC fingerprinting of methanolic extract of three medicinal plants, Int Res J Pharm. 4, 123-126, 2013.
Govindarajan VS. Pepper-chemistry, technology, and quality evaluation. CRC. Crit Rev Food Sci Nutr, 115-250, 1977.
Inder PS, Sandip B, Bharate AS, Kamlesh, KB. Fate of embelinin pappalyadi yoga, an ayurvedicoral contraceptive: structure of embelin-borex complexand evaluation of anti-fertility activity. Indian J Chem. 46, 320-325, 2007.
Kavirar Shastri A, Susruta Samhita of Maharsi Susruta, Edited with Ayurveda-Tatva-Sandipika Teeka, Mishrakadhyaya, Chaukhambha Sanskrit Sansthan, Varansi, Part-I, Sutta Sthana, pp. 146, 2005.
Kirtikar KR, Basu BD. Kirtikar and Basu’s Illustrated Indian Medicinal Plants, 3rd edition. Indian Medical Science Series, Sri Satguru Publications: Delhi, India, 2000.
Mukherjee PK. Quality control of herbal drugs, 1st Edition. Business Horizons, Pharmaceutical Publishers, Delhi, pp. 561-570, 2002.
Pei YQ. A review of pharmacology and clinical use of piperine and its derivatives and uses. Epilepsia. 24, 177-181, 1983.
Satyavati GV, Gupta AK, Tandon N. Medicinal Plants of India, Indian Council of Medical Research (ICMR): New Delhi, India, 1987.
Sharma PC, Yelne MB, Dennis TJ. Database of medicinal plants used in Ayurveda, Piper longum, 1st Edition. Documentation and Publication Division: CCRAS New Delhi, India, 2005.
Ujjaliya NBL, Vivek P, Remadevi R. A comparative phytochemical screening of roots and stem of Piper longum Linn. Int J Res Ayurveda & Pharm. 3, 67-69, 2012.
Wagner H, Bladt S. Plant drug analysis (A Thin Layer ChromatographyAtlas), 2nd Edition. Springer-Verlag: Berlin, Heidelberg, New York, 1996.









 This work is licensed under a
This work is licensed under a