Shaikh Mohammed Ishaque A. Hamid1, Amit Naik2
1M.D.Medicine (Z.V.M.Unani Medical College & Hospital, Pune); M.Sc. Pharmaceutical Medicine (M.U.H.S. Nashik& Seth GS Medical College & K.E.M.Hospital, Mumbai; D.E.M.S. & C.C.C.P.M. (Ruby Hall Clinic,Pune), MS, India.
2Amit Naik, Assistant Professor. UDIRT, MUHS, NASHIK, MS, India..
ORIGINAL RESEARCH ARTICLE
Volume 3, Issue 3, Page 132-136, September-December 2015.
Article history
Received: 1 December 2015
Revised: 18 December 2015
Accepted: 25 December 2015
Early view: 29 December 2015
*Author for correspondence
E-mail: [email protected]
Background: Package Insert (PI) contains detailed information about indications, warnings, precautions, side effects, dosage, administration, and clinical pharmacology. Package insert must bear adequate information for its use including indications, effects, dosages, routes, methods, & frequency duration of administrations &any relevant hazards, contraindications, side-effects & precautions under which practitioners licensed by law to administer the drug can use the drug safely &for the purposes for which it is intended including for all purposes for which it is advertised or represented.
Material & Methods: 200 Package inserts of allopathic parenteral & oral medicines of different pharmaceutical companies were randomly collected from pharmacists. Out of which 100 package inserts were of oral formulations, 100 were of parenteral formulations. Qualitative and Quantitative analysis was done on the basis of criteria given by WHO and FDA.
Results: The package inserts of oral formulations have been significantly better in all the aspects compared to the parenteral formulations.
Conclusion: Oral formulations are mainly used directly by the patients themselves and they don’t know the details about medical terminologies and concepts, whereas the parenteral formulations are mainly used by the doctors directly and they do not require the initial information and the details about the drug or formulation. The difference may be due to the package inserts of different pharmaceutical companies.
Key words: Allopathic, Oral, Parenteral, Package Insert, Completeness.
INTRODUCTION
Package Insert (PI) contains detailed information about indications, warnings, precautions, side effects, dosage, administration, and clinical pharmacology. PI is also referred to as “professional labelling” because it is written primarily for physician & pharmacist (William, 2006).
In USA, package inserts for only certain drugs such as oral contraceptives, antidepressants, etc., are required to contain patient-specific information. In the European Union, drug packages contain ‘package leaflets’ that are directed primarily to the patients. The text of package leaflets is based on the European Medicines Agency (EMEA) approved labelling information included in the Summary of Product Characteristics (SPCs), which are meant for healthcare professionals (FDA, 2006).
Package insert must bear adequate information for its use including indications, effects, dosages, routes, methods, & frequency duration of administrations &any relevant hazards ,contraindications ,side-effects &precautions under which practitioners licensed by law to administer the drug can use the drug safely &for the purposes for which it is intended including for all purposes for which it is advertised or represented. To present information in uniform manner FDA has issued labelling policies &format for Package insert (1) description (2) actions (3) indications (4) contraindications (5) warnings (6) precautions (7) ADR (8) dosage & administration(9) over-dosage (10)how supplied. Optionally P.I. may contain (11) Animal pharmacology & toxicology (12) Clinical studies (13) References (14) Other special warnings (Lachman et al., 2004).
Oral instructions alone are not enough; one-third of patients have been found unable to recount instructions immediately on leaving the consulting room. Lucid and legible labelling of containers is essential, as well as patient-friendly information leaflets, which are increasingly available via doctors and pharmacists and as package inserts (Bennet and Brown, 2003).
Specimen of the carton, labels, package insert that will be adopted for marketing the drug in the country shall be got approved from the Licensing Authority before the drugs is marketed (Drugs & Cosmetic Rules, 2005).
Product labels and package inserts should be understandable to the consumer or patient. The package information should include all necessary information on the proper use of the product. The following elements of information will usually suffice: (1) name of the product (2) quantitative list of active ingredient (3) dosage form (4) indications (5) dosage (if appropriate, specified for children and the elderly) (6)General guidelines for methodologies on research and evaluation of traditional medicine (7) mode of administration (8) duration of use (9) major adverse effects, if any (9)over dosage information (10) contraindications, warnings, precautions and major drug interactions (11) use during pregnancy and lactation (12)expiry date (13) lot number (14) holder of the marketing authorization. (15)Identification of the active ingredient(s) by the Latin botanical name (WHO, 2000).
Prescribers are also likely to depend on product information provided by pharmaceutical companies, something not unusual even in developed countries (Rajan, 2008).
However, product information provided by pharmaceutical companies in India has been determined to be far from adequate and not conforming with the WHO recommendations (Prosser, 2003).
Package inserts by virtue of being amenable to strict regulations and being readily available with the drug product can serve as reliable and accurate sources of drug information (Lal, 1996 & Joubert,1984).
Hence, a decision was taken to conduct a study, to evaluate the presentation and completeness of clinical information on package inserts of drug products marketed by pharmaceutical companies in India (Joubert, 1984).
This project includes the assessment of package inserts of Allopathic oral & parenteral medicines randomly collected from various medical shops of Nashik to ascertain the completeness of package inserts according to criteria’s given by FDA.
This project is very Important because 1- In recent years, there has been increase in length, detail, complexity of prescription drug labelling .making it harder for health care practitioners to find specific information. & to get the most critical information &In India there are no specific guidelines for determining content’s of P.I. Today burning issue is in each year number of preventable AE (Adverse Events) occurs many as a result of confusing medicinal information. In some previous years, information in P.I. became more complex &specific information is often difficult to locate. Community would get benefited in the following manner from this project. Improved P.I. can act to educate & guide the prescriber about all pharmacological facts of pharmaceutical agent at the various stages of its use.Improved P.I.s can help to avoid misuse of drugs. It will help to formulate the guidelines which are the need of the time.
MATERIALS AND METHODS
200 Package inserts of allopathic parenteral & oral medicines of different pharmaceutical companies are randomly collected from pharmacists. Out of which 100 package inserts are of oral formulations, 100 are of parenteral formulations Qualitative and quantitative analysis is done on the basis of criteria given by WHO and FDA.
For quantity assessment percentage of information present in individual package insert will be calculated. Each PI is scored as percentage of information out of 114 points for allopathic oral & parenteral formulations separately. Accordingly PIs will be divided into four groups depending upon their individual scores as follows:
• Grade I=up to 25% information
• Grade II=26 to50% information
• Grade III= 51 to 75% information
• Grade IV= 76 to 100 % information
Quality assessment will be done under nine categories containing 57 elements. Each category has its maximum points depending upon number of elements present & each element has two points such as;
1) Drug & Language (8 elements 16 points)
2) Composition, indication, Dosage (6 elements 12 points)
3) Pharmacodynamic (6 elements 12 points)
4) Pharmacokinetics (12 elements 24 points)
5) Metabolism (5 elements 10 points)
6) Dose adjustment/compliance (3 elements 6 points)
7) ADR/toxicity (4 elements 8 points)
8) Precaution/contraindications (6 elements 12 points)
9) Extra points (7 elements 14 points)
For CPCI each category will be analysis for all selected PI by percentage information, range & 95 % confidence interval.
Each element will be scored as 0=Absent, 1=Inadequate, 2=Adequate information &analyzed by Mean & number of 0’s, 1’s & 2”s present.
Calculation
Z TEST for difference between two proportions used for comparison between PI of allopathic oral and parenteral formulations, to find out which PI is more informative.
RESULTS
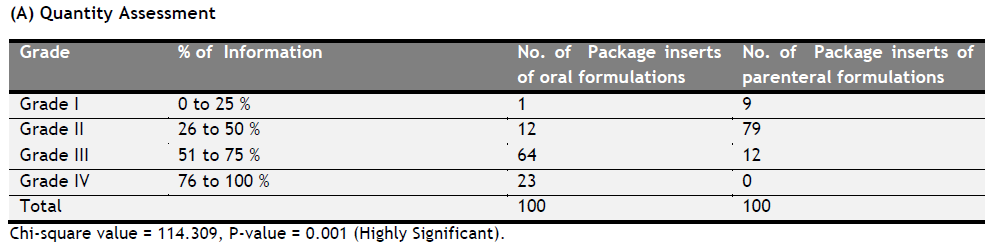 |
Table 1. (A) Quantity Assessment. Click here to view full image |
Significantly higher proportion of package inserts of oral formulations have higher grades compared to the package inserts of parental formulations.
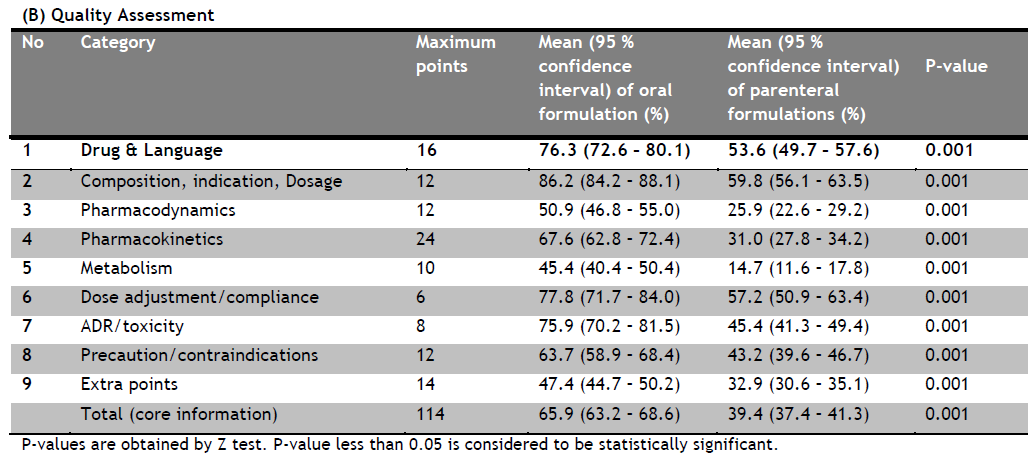 |
Table 2. (B) Quality Assessment. Click here to view full image |
The package inserts of oral formulations have been significantly better in all the aspects compared to the parenteral formulations.
DISCUSSION
There were few studies done all over the world. Some of them are as follows. A study was conducted in Saudi Arabia in which 37 PI of generic products registered in Saudi Arabia and manufactured in the Middle East were assessed, the result was substantial disagreement in information between generic package inserts versus the British National Formulary and the package insert of the brand product marketed in Saudi Arabia (Nicole & Khalid, 2006).
A study was conducted in Germany to improve readability and comprehensiveness of package inserts, in 73.5% of cases, the daily maximum dose was missing, similarly other different deficiencies like storage, temperature, high dose, dosage schedule were found (Fuchs, 2006).
A study was conducted in Germany for the purpose of availability and comprehensibility of the information contained on package inserts by giving a questionnaire containing 15 questions referring to the package insert contents was developed for a written survey (Fuchs, 2007). Package inserts accompanying allopathic drug products marketed by pharmaceutical companies in India were collected. The Aim was to assess the presentation and completeness of clinically important information provided in the currently available package inserts in India. The findings indicated considerable improvement in package inserts since 1996. However, on critical evaluation it was revealed that clinically important information was not well presented and was often incomplete. Information with regard to pediatric and geriatric use was present in only 44% and 13% of the package inserts, respectively. Only five of the inserts had information on the most frequent adverse drug reactions associated with the drug. Also, information on interactions and over dosage was often missing (Shivkar, 2009).
A study was conducted in Palestine whose objective was to evaluate the perception of health professionals and industry personnel towards the appropriate use of oral drops and to assess their package inserts with regard to presence of proper instructions for use and storage. The results were: The majority of physicians and pharmacists (73.3% and 71.2%) thought that oral drops can be delivered from the dropper directly into the patient’s mouth. 60.8% of physicians and 54.3% of pharmacists thought that the dropper should be clamped vertically when oral drop are dispensed. 72.7% of industry personnel agreed that the angle of inclination affects the drop size. Many of these personnel said their companies did not perform the recommended tests for dose uniformity and calibration. Instructions for storage and proper use were not available in package inserts of many oral drop products in Palestine (Zaid & Rahami, 2010).
Study was done in Palestine the objective was to explore how patient information leaflets influence patient anxiety and adherence. The results found were the study group comprised 200 patients. The patient information leaflet was read by 103 of them (51.5%). A higher educational level and a chronic medication were associated with reading the leaflet (P= 0.02 and 0.01 respectively). In 36 (34.9%), an increase in anxiety was reported after reading the leaflet. Among those who read the leaflet, 9.7% had decreased adherence. Patients who stated that reading the leaflet caused anxiety were more likely to reduce their use of the medication-7/36 (19.5%) vs. 3/67 (4.5%), P= 0.01(Vinker, 2010).
Keeping these studies in mind, we can discuss this project.
Out of 100 oral package inserts 1 P.I. is having Grade I (up to 25% information), 12 are having Grade II(26 to 50 % information ),64 are having Grade III(51 to 75 % information) ,23 can be classified under Grade IV(76 to 100 % information). Whereas Out of 100 parenteral package inserts 09 P.I. are having Grade I (up to 25% information), 79 are having Grade II(26 to 50% information), 12are having Grade III(51 to 75 % information) and there is no P.I. which can be classified under Grade IV(76 to 100 % information).
Most of the P.I. are having adequate information related with the Pharmacokinetics whereas there is very little information given related with the dose adjustment/ compliance followed by ADR/ toxicity.
The package inserts of oral formulations have been significantly better in all the aspects compared to the parenteral formulations. It may be because the oral formulations are mainly used directly by the patients themselves and they don’t know the details about medical terminologies and concepts, whereas the parenteral formulations are mainly used by the doctors directly and they do not require the initial information and the details about the drug or formulation. The difference may be due to the package inserts of different pharmaceutical companies.
The FDA rules & regulations are not followed by many pharmaceutical companies so; most of the package inserts are not complete.
CONCLUSION
Hence it can be concluded that Most of the P.I. are having adequate information related with the Pharmacokinetics whereas there is very little information given related with the dose adjustment/ compliance followed by ADR/ toxicity. The package inserts of oral formulations have been significantly better in all the aspects compared to the parenteral formulations. It may be because the oral formulations are mainly used directly by the patients themselves and they don’t know the details about medical terminologies and concepts, whereas the parenteral formulations are mainly used by the doctors directly and they do not require the initial information and the details about the drug or formulation. The difference may be due to the package inserts of different pharmaceutical companies. The FDA rules & regulations are not followed by many pharmaceutical companies so; most of the package inserts are not complete.
Limitations of the study
As sample size increases precision increases. In this study sample size is small and this is not a comparative or parallel group study, and it would have been better if the study include package inserts of only parenteral or oral formulations comprising of various groups like pediatric, adult, etc.
Scope for further study and recommendations
Parallel group study can be designed with large sample size.
ACKNOWLEDGEMENT
I acknowledge to Dr. Abdul Hamid, Dr. Ismail, Mother, and Dr. Sangle for their support.
CONFLICT OF INTEREST
None declared.
REFERENCES
Bennett PN, Brown MJ. Topics in Drug Therapy: Clinical Pharmacology; Churchill Livingstone, Sydney. 2003: 20.
Dikshit RK, Dikshit ND. What information is available on request from drug advertisers BMJ . 1996; 313:855-6.
Drugs & Cosmetic rules (amended 30th June, 2005): 239.
FDA. Requirements on content and format of labeling for human prescription drug and biological products. Federal Regulation. 2006; 71:3921-97.
Fuchs, J, &Hippius, M. Inappropriate dosage instructions in package insert, Patient education counsil; 2007. 67(1-2):157-68.
Fuchs, J, Hippius, M, & Schaefer, M. Analysis of German package inserts, International journal clinical pharmacology Therapeutics, 2006. 44(1):8-13.
Joubert PH, Skene D. Attitudes of private medical practitioners towards package inserts and other drug information sources. South African Medical Journal. 1984; 66:3067.
Lal A, Sethi A. Drug package inserts in India. Ann Pharmacotherapy 1996; 30:1041.
Leon L,Joseph A , kang, herbert A, Lieberman . The Theory &Practice of industrial pharmacology, 2004: 868.
Nicole, G &Khaled, AH. Assessment of prescribing information for generic drugs manufactured in the middle east and marketed in Saudi Arabia, Ann Saudi Med, 2006 (3):192-99.
Prosser H, Almond S, Walley T. Influences on GPs′ decision to prescribe new drugs-the importance of who says what. Family Practice; 2003; 20:618.
Rajan MS, Sreedhar, Khan SA, Thiyagu R, Rao PG. Information seeking behaviour of clinicians in a semi urban town in southern India. Journal of Clinical Diagnosis. 2008; 2:1069-1073.
Shivkar YM. Clinical Information in drug package inserts in India. Journal of Post Graduate Medicine. 2009. 55(2);104-107.
Vinker S, Eliyahu V, Yaphe . International journal of clinical pharmacology &therapeutics. 2010; 48(12):854-9.
WHO Geneva. Operational Guidelines for Ethics Committee that review biomedical research. 2000: 25.
Zaid, Al-Ramahi , Abu Ghoush. International journal of Clinical Pharmacology & Therapeutics. 2010;48(12):854-859.









 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.