Lakshmi BVS *, Divya V.
Department of Pharmacology, Malla Reddy College of Pharmacy, Dhulapally, Maisammaguda, Secunderabad-500014, TS, India.
ORIGINAL RESEARCH ARTICLE
Volume 2, Issue 3, Page 148-153, September-December 2014.
Article history
Received: 6 December 2014
Revised: 20 December 2014
Accepted: 28 December 2014
Early view: 31 December 2014
*Author for correspondence
E-mail: [email protected]
Mob.: +919885324334
Background: The objective was to investigate the antiurolithiatic and antioxidant activity of ethanolic extract of Zingiber officinale (EZO) on ethylene glycol-induced urolithiasis.
Materials and methods: EZO was assessed for its curative and preventive action in urolithiasis. In preventive treatment, the EZO is given from 1st day to 28th day, while in curative regimen; the EZO was given from 15th day to 28th day. Various renal functional and injury markers such as urine volume, calcium, phosphate, uric acid, magnesium and urea were evaluated using urine, serum, and kidney homogenates. Antioxidant parameters such as MDA, GR, GSH and CAT were also estimated.
Results: The EZO treatment (both preventive and curative) increased urine output significantly compared to control. The EZO treatment significantly reduced urinary excretion of calcium, phosphate, uric acid, magnesium and urea. The increased deposition of stone forming constituents in kidneys of calculogenic rats were significantly lowered by curative and preventive treatment with EZO. Increased levels of MDA and decreased levels of GR, GSH and CAT were observed in calculi-induced animals compared to normal animals. The treatment with EZO (200 and 400mg/kg) and cystone produced significant results. This is in conformation with histopathology results.
Conclusion: These results suggest usefulness of ethanol extract of Zingiber officinale rhizome as an antiurolithatic agent.
Keywords: Antioxidant, ethylene glycol, Zingiber officinale, urolithiasis.
INTRODUCTION
Urinary calculi are the third prevalent disorder of the urinary system. Approximately 80% of these calculi are composed of calcium oxalate and calcium phosphate. Lithiasis is a malepredominant disorder, with a recurrence rate of 70–80% in males and 47–60% in females. Currently, no allopathic medications are available for urolithiasis. Surgery and calculus disruption is a common technique to treat calculi. But, these procedures are costly (Prasad et al., 2007).
Zingiber officinalis, Roscoe is a rhizome that is widely used as culinary herb and herbal remedy for some common ailments. It is commonly used as carminative, antipyretic, antiemetic in pregnancy and as anticancer adjunct (Evans and Trease, 1979). It is also used to treat motion sickness and is a diaphoretic agent. It contains about 1-2% of volatile oil and 5-8% of resinous matter, starch and mucilage. The volatile oil contains monoterpenes, sesquiterpenes and sesquiterpene alcohol zingiberol, gingerol and shagoals. Most of the pharmacologically active constituents reside in the volatile oils. Gingerols have cardio tonic (Koboyashi et al., 1988), analgesic, anti-inflammatory (Haw-Yaw et al., 2005), antipyretic (Yoshikawa et al., 1993) and antibacterial effects both in vitro and in vivo (Mascolo et al., 1989). Shagoal has antiemetic, antispasmodic, anxiolytic and anticonvulsant activity (Vishwakarma et al., 2002). Scientific reports show that it is also used for conditions such as anti-ulcerogenic (Al-Yahya et al., 1989), anti-diabetic (Ahani et al., 1981), anti-oxidant (Jeyakumar et al., 1999), anti-hepatotoxic (Omoniyi et al., 2006), adaptogenic and nephroprotective (Lakshmi et al., 2010) activities. Ginger has been reported to possess a potent anti-oxidant activity in vitro which reduces the oxidative stress in the body. In the present study, an effort has been made to establish the scientific validity of the antiurolithiatic activity of Zingiber officinale rhizome extract using ethylene glycol-induced urolithiasis using male Wistar albino rats.
MATERIALS AND METHODS
Adult male albino rats of Wistar strain (150-200 g) were procured from National Institute of Nutrition, Hyderabad. For acute toxicity studies, albino mice of either gender (25-30 g) were also procured. The animals were acclimatized to standard laboratory conditions (temperature: 23 ± 2°C) and maintained on 12-hour light/dark cycle. They were provided with regular diet with free access to drinking water ad libitum for the period of 28 days. Institutional Animal Ethics Committee (IAEC) approval (Protocol no.: MRCP/CPCSEA/IAEC/2012-13/8) was obtained and care of the animals was taken as per guidelines of CPCSEA, Ministry of Social Justice and Empowerment, Government of India.
Chemicals
Ethylene glycol was obtained from Qualigen Fine Chemicals, Mumbai, India and cystone (Himalaya Health Care, india) was procured from the local market. All other chemicals and reagents used were of analytical grade.
Preparation of the ethanolic extract
The fresh rhizomes of ginger were taken, sliced and the slices obtained were shade-dried, pulverized and passed through a 20-mesh sieve. The dried, coarsely powdered material was extracted with ethanolic extract using Soxhlet apparatus at a temperature below 60◦C for 24 hours. The solvent was evaporated under vacuum, which gave semisolid mass (yield: 57%w/w) with respect to the dried powder. Oral suspensions containing 100mg/ml, 200mg/ml and 400 mg/ml of the ethanolic extract of ginger was prepared and used for the evaluation of anti-uroliathiatic effect.
Acute toxicity studies
Acute toxicity study was performed as per the OECD guideline (no. 423) using albino mice prior to the evaluation of antiurolithiatic activity. The EZO was tested using graded doses (50, 100, 500, and 2000 mg/kg) in mice.
Experimental design
Ethylene glycol-induced urolithiasis model was used to assess the antiurolithiatic activity in albino Wistar rats (Atmani et al., 2003). Animals were divided into nine groups containing six animals each. Group I served as control and received regular rat food and drinking water ad libitum. Ethylene glycol (0.75% v/v) in drinking water was fed to groups II–IX for 28 days to induce formation of renal calculi. Group III received standard antiurolithiatic drug, cystone (750 mg/kg b.w.; p.o.). Groups IV, V, and VI served as curative regimen (CR) and received EZO 100 mg/kg, 200 mg/kg, and 400 mg/kg body weight respectively from 15th to 28th day. Groups VII, VIII, and XI received EZO 100 mg/kg, 200 mg/kg, and 400 mg/kg body weight respectively from the 1st day till the 28th day and served as preventive regimen (PR). All extracts and standard were given once daily by oral route
Analysis of urine and serum
At the end of treatment, all animals were kept in individual metabolic cages and 24-hour urine samples were collected and measured on the 28th day. Animals had free access to drinking water during the urine collection period. A drop of concentrated hydrochloric acid was added to the collected urine before being stored at 4°C. The urine was analyzed for calcium, magnesium, phosphate, urea, and uric acid.
On the 29th day, the animals were anesthetized with diethyl ether and blood was collected by cardiac puncture method. Serum was separated by centrifugation at 5,000 rpm for 20 minutes and analyzed for serum calcium, phosphate, magnesium, uric acid, urea and creatinine.
Kidney homogenate analysis
The abdomen was cut open to remove both kidneys from each animal. The Isolated kidneys were taken and washed in ice-cold physiological solution. The kidneys were fixed in 10% neutral buffered formalin, for further processing and staining with hematoxylin and eosin (H and E) for histopathological examination. The slides were examined under a light microscope to study the architecture of the kidney and calcium oxalate deposits. A sample of 100 mg of the dried kidney was boiled in 10 ml of 1N hydrochloric acid for 30 minutes and homogenized. The homogenate was centrifuged at 2000 rpm for 10 minutes and the supernatant was separated. The calcium, phosphate and uric acid content in the kidney homogenate were determined.
Tissue sample preparation
At the end of the experiment, animals were sacrificed with light ether anesthesia. Kidney tissues were separated and washed with phosphate buffer saline (0.05M, PH7.4). Then kidney was later minced into small pieces and homogenized in ice cold phosphate buffer saline (0.05M, PH7.4) using tissue homogenizer to obtain 1:9 (w/v) (10%) whole homogenate. A part of the kidney homogenate was taken and mixed with equal volume of 10% Trichloroacetic acid (TCA) for the estimation of malondialdehyde. Homogenate was centrifuged using Remi cool centrifuge at 8000 rpm for 30 mins. The supernatant was separated and used for estimation of anti-oxidant levels of different enzymes i.e. Catalase, glutathione reductase and reduced glutathione are estimated.
Statistical analysis
Results were expressed as mean ± SEM. Differences among data were determined using one-way ANOVA followed by Dunnett’s multiple comparison tests (Graph pad Prism software for Windows, Version 2.03.1998). P < 0.05 was considered to be statistically significant.
RESULTS
In the acute toxicity study, no signs of toxicity were found upto the dose of 2000 mg/kg body weight. Hence 1/20th, 1/10th and 1/5th doses i.e. 100 mg/kg, 200 mg/kg and 400 mg/kg have been fixed as ED50 for present study.
Table 1 depicts the urinary biochemical data that were obtained at the end of the experiment in each group. In the present study, EZO significantly (P < 0.05) has increased the urine volume at doses of 200 and 400 mg/kg indicating its diuretic activity. Chronic administration 0.75% (v/v) ethylene glycol aqueous solution to Wistar rats resulted in hyperoxaluria. There was an increase in urinary calcium, phosphate, uric acid, magnesium and urea in calculi induced animals [Table 1, group II]. However, supplementation with EZO(200 and 400 mg/kg) significantly (P <0.05) inhibited these changes in urinary calcium, phosphate, uric acid, magnesium and urea excretion dose-independently in both curative and preventive regimen [Table 1, group IV-IX]. Renal stone induction caused impairment of renal functions of the untreated rats as evident from the makers of glomerular and tubular damage, i.e. elevated serum creatinine, uric acid and urea. These markers were significantly (P <0.05) reduced in the animals which were treated with EZO in a dose-dependent manner. The serum calcium, inorganic phosphate and magnesium were significantly increased (P < 0.05) in calculi-induced animals compared to Group I [Table 2, Group II] indicating marked renal damage. However treatment with EZO (200 and 400mg/kg) significantly (P < 0.05) lowered the elevated serum level of calcium, inorganic phosphate and magnesium in both curative and preventive regimens [Table 2, Group IV-IX]. The calcium, phosphate and uric acid levels were significantly elevated in kidney homogenate of the calculi- induced animal group (group II) compared to group I [Table 3]. The EZO (400 mg/kg) and cystone treatment significantly (P <0.05) diminished the levels of all parameters mentioned above in both regimens. Low dose of EZO (100 and 200 mg/kg) exhibited significant reduction in calcium and uric acid in kidney homogenate except phosphate level. Ethylene glycol treatment significantly (P <0.05) increased the MDA levels and decreased the GR, GSH and CAT levels in calculi-induced animals compared to the normal animals [Table 4, Group II].The treatment with EZO (200 and 400mg/kg) produced significant (P <0.05) reduction in MDA and improved the level of antioxidant enzymes like GSH compared to group II [Table 4, group l—IX]. The elevated level of CAT was significantly (P <0.05) maintained by the treatment with EZO 400 in both curative and preventive treatment. [Table 4, Group VI, IX]. Histopathalogical analysis revealed no calcium oxalate deposits or other abnormalities in the nephron segment of the vehicle treatment group [Fig.1a]. On the other hand, several calcium oxalate deposits inside the tubules and dilation of the proximal tubules along with intestinal inflammations were observed in the renal tissue of urolithiatic rats [Figure 1b]. The number of calcium oxalate deposits in the tubules of EZO treated rats [group IV-IX (1d- 1 i.)] and cystone treated rats [group III (1c)] were less than group II (1b).
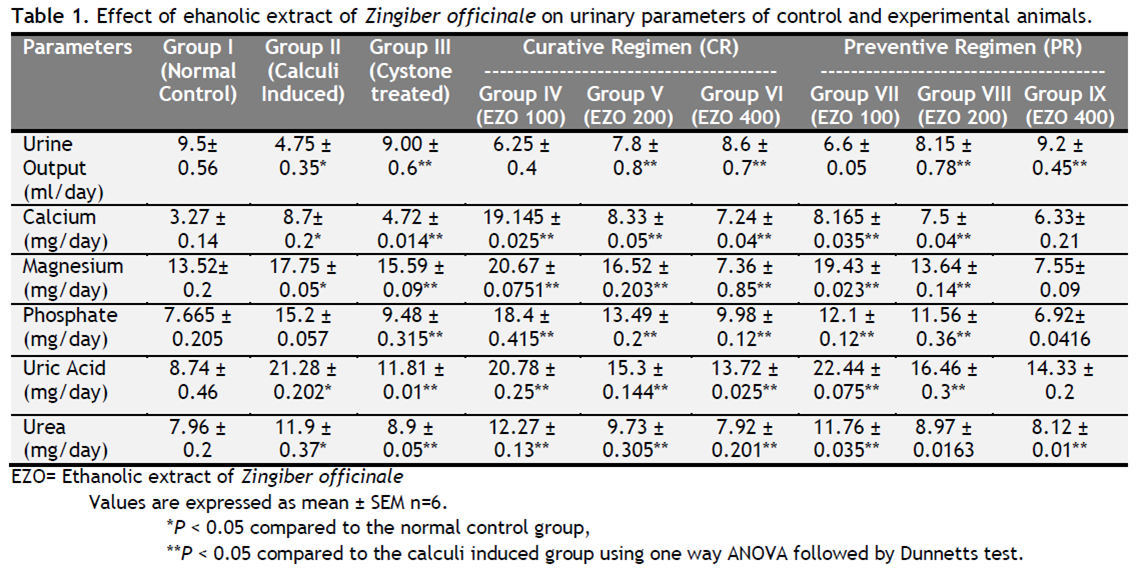 |
Table1. Effect of ehanolic extract of Zingiber officinale on urinary parameters of control and experimental animals. Click here to view full image |
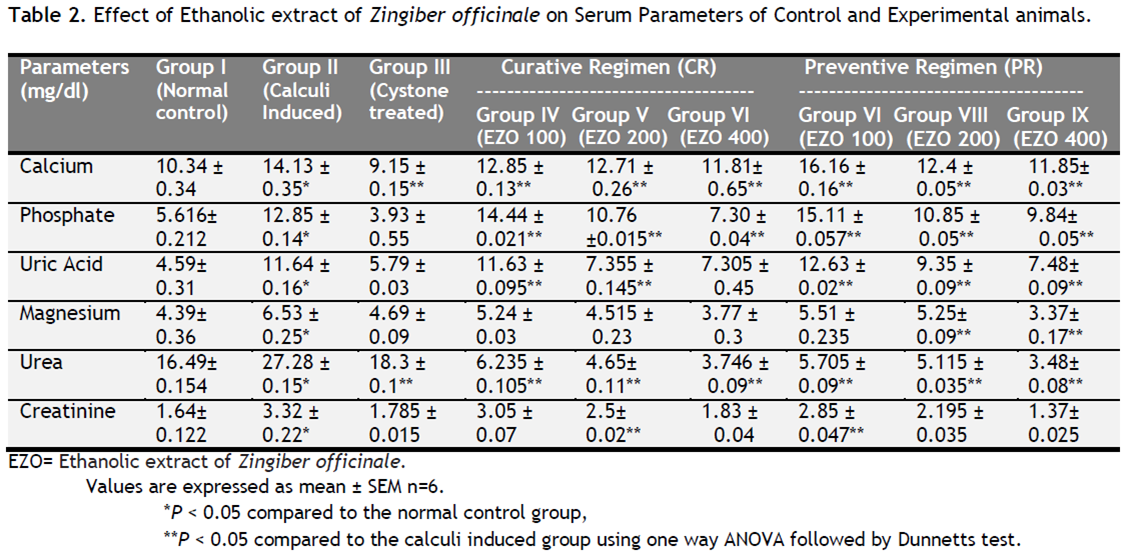 |
Table 2. Effect of Ethanolic extract of Zingiber officinale on Serum Parameters of Control and Experimental animals. Click here to view full image |
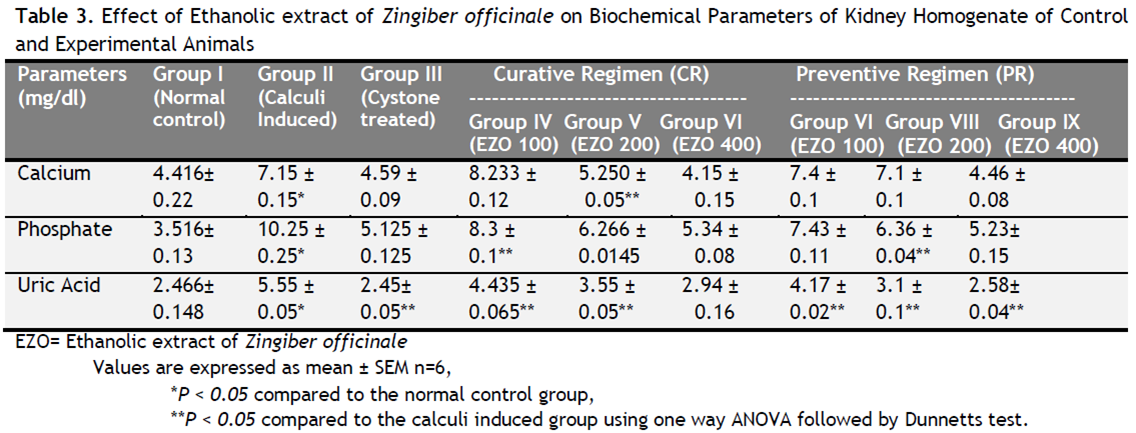 |
Table 3. Effect of Ethanolic extract of Zingiber officinale on Biochemical Parameters of Kidney Homogenate of Control and Experimental Animals. Click here to view full image |
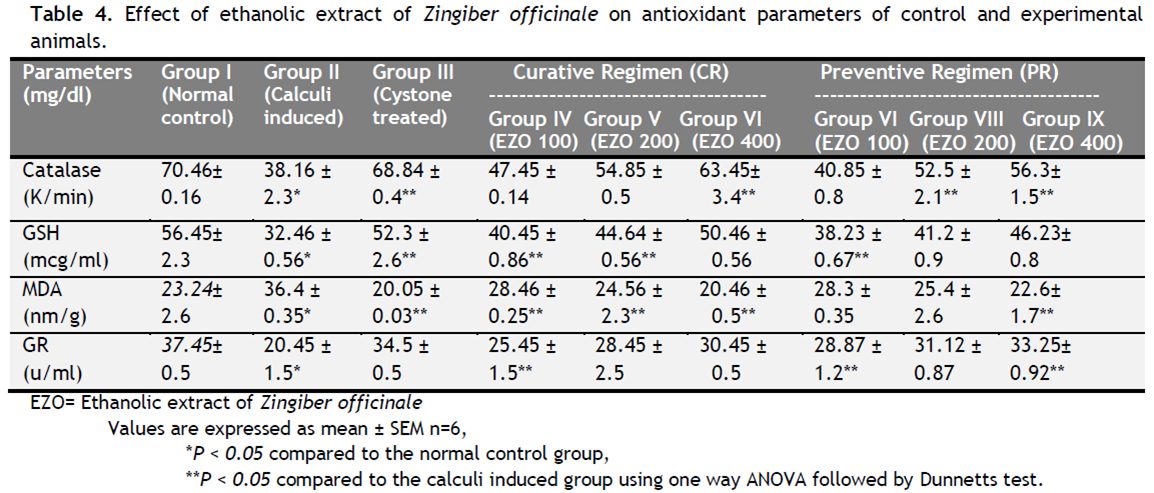 |
Table 4. Effect of ethanolic extract of Zingiber officinale on antioxidant parameters of control and experimental animals. Click here to view full image |
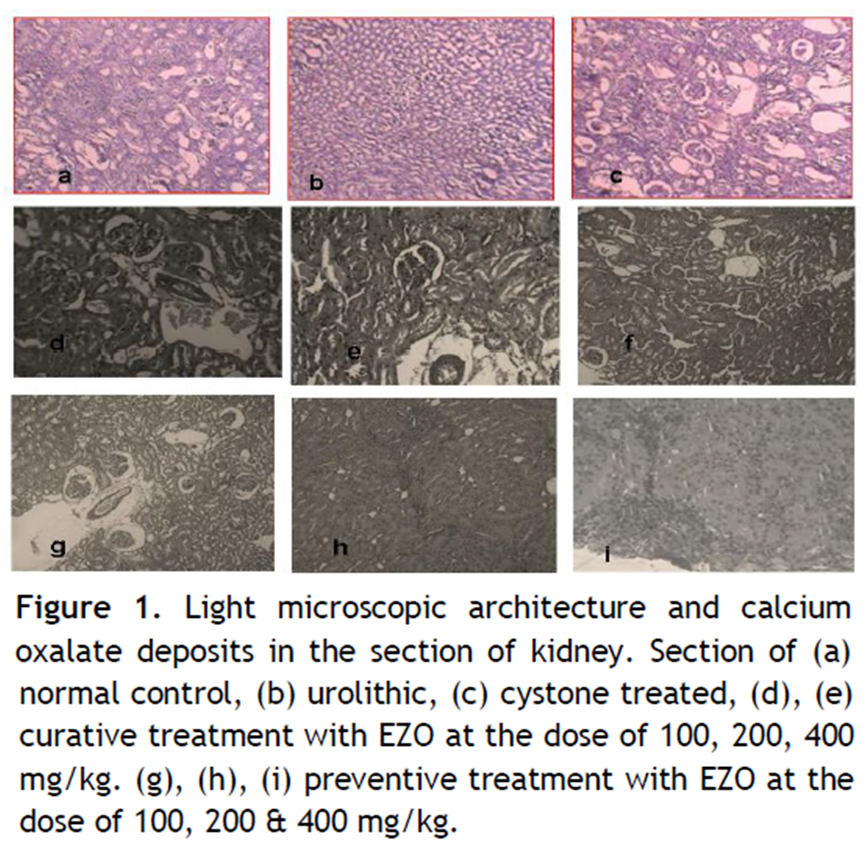 |
Figure 1. Light microscopic architecture and calcium oxalate deposits in the section of kidney. Section of (a) normal control, (b) urolithic, (c) cystone treated, (d), (e) curative treatment with EZO at the dose of 100, 200, 400 mg/kg. (g), (h), (i) preventive treatment with EZO at the dose of 100, 200 & 400 mg/kg.. Click here to view full image |
DISCUSSION
A number of renal pathological diseases, including calcium oxalate kidney stones, have resulted due to the oxalate-induced damage to renal cells. Elevated levels of oxalate are responsible for the toxic effects on the renal epithelial cells via alteration in membrane integrity, generation of reactive oxygen species, and depleted source of anti-oxidant enzymes (Miller et al., 2000).
In the present study, rats were selected to induce renal stone because their urinary system resembles that of humans. Evidence in previous studies indicated that in response to the 28-day period of ethylene glycol (0.75%, v/v) administration, young albino rats form renal calculi composed mainly of calcium oxalate, minute amounts of calcium phosphate and uric acid stone (Kurien and selvam, 1989). Ethylene glycol increases the risk of urolithiasis by increasing urinary levels of stone constituents (calcium, oxalate, phosphate and uric acid) and facilitating an optimal environment for stone. Ethylene glycol increases oxalate production by way of increasing substrate availability which induces the activity of oxalate synthesizing liver enzyme, glycolate oxidase (Robertson et al., 1985). In view of its medicinal use as an antiurolithic, Zingiber officinale extract was studied to evaluate its potential to prevent calcium oxalate urolithiasis. To our knowledge this is the first study to show the anti-urolithiatic effect of EZO in ethylene glycol-induce urolithiasis.
The present study showed an increase in urine output of EZO treated animals which dilute the concentration of urinary electrolytes. As a result, calcium and phosphorous are flushed out via the urine and there are lesser chances of precipitation, decreased formation as well as the growth of urinary stone. The excretion of calcium was progressively increased in calculi-induced animals which is consistent with the previous reports (Divakar et al., 2010). Most calculi in the urinary system arise from a common component of urine such as calcium (CaOx) and hypercalciuria, representing upto 80% of analyzed stones (Lemann et al., 1991). Increased urinary calcium is a factor favouring the nucleation and precipitation of calcium oxalate or apatite (calcium phosphate) from urine and subsequent crystal growth. However, EZO lowered the levels of calcium excretion, which is beneficial in preventing calculi formation.
Increased urinary inorganic phosphate excretion observed on ethylene glycol administration along with oxalate stress seems to provide an environment appropriate for stone formation by forming calcium phosphate crystals, which eventually induces calcium oxalate deposition (Kohri et al., 1988). Treatment with EZO restored inorganic phosphate level, thus reducing the risk of stone formation. Magnesium, a potent inhibitor of CaOx crystallization in vitro, binds to oxalate to form a soluble complex; consequently reducing the concentration available for CaOx precipitation. Magnesium deficiency accelerates the deposition of renal tubular calcium oxalate in rats. Experiments in animal models have shown protection against CaOx kidney stones. However, treatment with EZO significantly reduced the level of magnesium in urine and serum and significant elevation in ethylene glycol control animals.
The glomerular filtration rate decreases in urolithiasis due to the obstruction to the outflow of urine by stones in the urinary system and waste products such as urea and uric acid get accumulated in blood. This indicates marked damage of kidney. The uric acid levels decreased after treatment with EZO and cystone, thereby hastening the process of dissolving the preformed stones and prevention of new stone formation in the urinary system (Purnima et al., 2010).
It has been postulated from several in vitro and in vivo studies that high levels of phosphate may have a detrimental effect on renal architecture mediated through intracellular oxidative stress (Bashir et al., 2010). It was observed in the present study that administration of ethyl glycol increased MDA content of kidneys and decreased activity of the antioxidant enzymes in the kidneys. The EZO treatment protected against the changes associated with oxidative stress. Several studies have shown that crystal formation results in cell damage and cell detachment from the basement membrane and the released degradation products further promote nucleation of crystals (Khan and Hackett, 1991). Renal epithelial injury promotes crystal retention, as epithelial injury exposes a variety of crystal adhesion molecules on epithelial surfaces (Bijarnia et al., 2008).
Histopathological changes also support the above results. Administration of EZO to ethylene glycol rats prevented super saturation of calcium oxalate and thus decreased their deposition in renal tubules due to active compound present in the extract. Microscopic examination of kidney section derived from nephrolithiatic rats showed intra tubular and interstitial crystal deposits. In the present investigation histopathological showed maximum prevention of crystal deposition in the experimental study.
CONCLUSION
The results indicate that administration of EZO reduced and prevented the growth of urinary stones. It also seems that the treatment effect is more effective than its preventive effect. Further experimental and clinical studies are required to elucidate the chemical constituents of the extract and the mechanism(s) that are responsible for the pharmacological activities.
ACKNOWLEDGEMENTS
The authors are thankful to the management of Malla Reddy College of Pharmacy, for providing the chemicals and required facilities to carry out the research work.
CONFLICT OF INTEREST
None declared.
REFERENCES
Akhani SP, Vishwakarma SL, Goyal RK. Anti-diabetic activity of Zingiber officinale in streptozotocin-induced type I diabetic rats. J Pharm Pharmacol. 56, 101-105, 1981.
Al-Yahya MA, Rafatullah S, Mossa JS, Ageel AM. Gastroprotective activity of ginger Zingiber officinale rosc., in albino rats. Am J Chin Med. 17, 51-56, 1989.
Atmani F, Slimani Y, Mimouni M, Hacht B. Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. BJU Int. 92, 137-140, 2003.
Bashir S, Gilani A H, Siddiqui AA, Pervez S, Khan SR, Sarfaraz NJ, et al. Berberis vulgaris root bark extract prevents hyperoxaluria induced urolithiasis in rats. Phytother Res. 24, 1250-1255, 2010.
Bijarnia RK, Kaur T, Aggarwal K, Singla SK, Tandon C. Modulatory effects of N-acetylcysteine on hyperoxaluric manifestations in rat kidney. Food Chem Toxicol. 46, 2274-2278, 2008.
Divakar K, Pawar AT, Chandrasekhar SB, Dighe SB, Divakar G. Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol. 48, 1013-1018, 2010.
Evans W C and Trease GE, Volatile oils and resins, Pharmacognosy (11th ed. Balliere, Tindale), 475, 1979.
Haw-Yaw Young, Yen-Lin Luo, Hao-Yuan Cheng, Wen-Chiuan Hsieh, Jung-Chun Liao, Wen-Huang Peng. Analgesic and anti-inflammatory activities of [6]-gingerol. J Ethnopharmacol. 96, 207-210, 2005.
Jeyakumar SM, Nalini, Menon VP. Antioxidant activity of ginger (Zingiber officinale Roscoe) in rats fed with a high fat diet. Medical Sci Res. 27, 341-346, 1999.
Khan SR, Hackett RL. Retention of calcium oxalate crystals in renal tubules. Scanning Microsc. 5, 707-712, 1991.
Kobayashi M, Ishida Y, Shoji N, Ohizumi Y. Cardiotonic action of (8)-gingerol, an activator of Ca 2+ - pumping adenosine triphosphate of sarcoplasmic reticulum, in guinea pig atrial muscle. J Pharmacol Exp Ther. 246, 667-673, 1988.
Kohri K, Garside J, Blacklock NJ. The role of magnesium in calcium oxalate urolithiasis. Br J Urol. 61, 107-115, 1988.
Kurien TB, Selvam R. Induction of lipid peroxidation in calcium oxalate stone formation. Indian J Exp Biol. 27, 450-453, 1989.
Lakshmi BVS, Sudhakar M. Attenuation of acute and chronic restraint stress- induced perturbations in experimental animals by Zingiber officinale Roscoe. Food Chem Toxiocol. 48, 530-535, 2010.
Lakshmi BVS, Sudhakar M. Protective Effect of Zingiber officinale on Gentamicin- induced nephrotoxicity in rats. Internat J Pharmacol. 6, 58-62, 2010.
Lemann J, Worcestor EM, Gray RW. Hypercalciuria and stones. Am J Kidney Dis. 27,386-391, 1991.
Mascolo N, Jain R, Jain SC, Capasso F. Ethno pharmacologic investigation of ginger (Zingiber officinale Roscoe). J EthnoPharmacol. 27, 129-140, 1989.
Miller C, Kennington L, Cooney R, Kohjimoto Y, Cao L C, Honeyman T, et al. Oxalate toxicity in renal epithelial cells: Characteristics of apoptosis and necrosis. Toxicol Appl Pharmacol. 162, 132-141, 2000.
Omoniyi K, Yemitan, Matthew C, Izegbu. Protective effects of Zingiber officinale (Zingiberaceae) against carbon tetrachloride and acetaminophen-induced hepatotoxicity in rats. Phytother Res. 20, 997, 2006.
Prasad K, Sujatha D, Bharathi K. Herbal drugs in urolithiasis – a review. Pharmacognosy Res. 1, 175-179, 2007.
Purnima A, Basavaraj C, Koti AH, Vishwanathswamy. Anti-urolithiatic and antioxidant activity of Minusops elengi on ethylene glycol induced urolithiasis in rats. Ind J Pharmacol. 42, 380-383, 2010.
Robertson WG, Peacock M, Selby PL. A multicenter trial to evaluate three treatments for recurrent idiopathic calcium stone disease: A preliminary report. In: Schwille PO, Smith LH, Robertson WG, Vahlensieck W, editors. Urolithiasis and Related Clinical Research. New York: Plenum Press; p. 545-548, 1985.
Vishwakarma SL, Pal SC, Kasture VS, Kasture SB. Anxiolytic and antemetic activities of Z. officinale. Phytother Res. 16, 621-626, 2002.
Yoshikawa M, Hatakeyama S, Chatani N, Nishino Y, Yamahara J. Qualitative and quantitative analysis of bioactive principles in Zingiberis rhizome by means of high performance liquid chromatography and gas and liquid chromatography: On the evaluation of zingiberis rhizome and chemical change of constituents during zingiberis rhizome processing. Yakugaku Zasshi. 113, 307-315, 1993.









 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.