Areeb Inamdar1*, Sahera Nasreen2
1Department of Biotechnology, Shivchhatrapati College, Aurangabad, MS, India.
2Department of Botany, Govt. Institute of Science, Aurangabad, MS, India.
SHORT COMMUNICATION
Volume 2, Issue 1, Jan-April 2014.
Article history
Received: 28 March 2014
Revised: 18 April 2014
Accepted: 25 April 2014
Early view: 28 April 2014
*Author for correspondence
E-mail: [email protected]
Mobile/ Tel.: +91 000000000
Keywords:
Amylase inhibitors
Enzyme
T. castaneum
H. armigera
Pest
Zea mays.
The present global scenario in context to agriculture productivity and loss explores the series of unpleasant surprising. The main objective the present study is to investigate the activity of amylase inhibitor’s (AI’s) from plant storage material i.e. seed, against amylase enzyme of insects Tribolium castaneum and Helicoverpa armigera. Amylase inhibitors was extracted from seeds of Cicer areitinum, Triticum aestivum, Pennisetum typhoides and Zea mays and tested against amylase of two larvae T. castaneun and H. armigera. The protein Quantitation of crude alpha amylase inhibitors was carried out and compared with Bovine serum albumin. The plant seed extracts showed inhibitory activity against amylase enzyme obtained from T. castaneum and H. armigera respectively. The maize amylase inhibitor (MAI’s) extract showed maximum inhibitory activity as compared to other source against amylase isolated from both organisms. The amylase inhibitor extracted from Z. mays showed 82.1 % and 85.12% inhibition against T. castaneum and H. armigera respectively. In the present study all four experimental seed samples posses’ potent insect amylase inhibitors. These inhibitors block the activity of insect’s gut amylase with varying percentage. Which is beneficial to control the pest in agricultural to avoid loss of agricultural yield.
INTRODUCTION
The present global scenario in context to agriculture productivity and loss explores the series of unpleasant surprising. Worldwide crop losses without the use of pesticides and other non-chemical control strategies is estimated and found to be about 70% of crop production, amounting to US $ 400 billion. The world wide pre-harvest loss due to insect pests, despite, the use of insecticides is 15% of total production representing over US $ 100 billion (Kratiger et.al., 1997). The annual cost of insect control itself amounts to US $ 8 billion, thus warranting urgent economical control measures. In order to feed the ever expanding population, crop protection plays a vital and integral role in the modern day agricultural production to minimize yield losses. To balance between the desired productivity level and loss during the cultivation or during harvest and post harvest condition, some concrete strategies are required to implement; because the gap between agricultural productivity and demand is day to day increasing. On the other hand the arable land is diminishing every year as it is diverted for industrial, residential, recreational and other human needs. Therefore, today we have the challenge to ‘produce more from less’ by developing biotechnological strategies.
Insect pest menace is the major factor that destabilizes crop productivity in agricultural ecosystems. One of the practical means of increasing crop production is to minimize the pest associated crop losses. The chemical control of insect pests is an effective option; most often it is expensive and depends mainly on the weather conditions. Extensive application of chemical pesticides not only builds up resistance in insect pests but also proves deleterious to the beneficial organisms such as pollinators, nutrient cyclers and natural pest-controlling agents owing to their non-selective properties (Lawrence et. al., 2002). Moreover, indiscriminate usage of pesticides exerts harmful effects on the environment and human health through food chain. As such, adoption of insect resistant cultivars has been considered as the most economic and eco-friendly strategy for pest management (Lawrence et. al., 2002).
The present study is concerned on the activity of amylase inhibitor’s (AI’s) from plant storage material i.e. seed. The following four sources were taken into consideration. Chick pea (Cicer areitinum), Wheat (Triticum aestivum), Millet (Pennisetum typhoides ) and Maize seeds (Zea mays). Various workers screened plant Amylase inhibitor against different organism (Buonocore et al., 1986; Hasenah et al., 2006)
MATERIAL AND METHODS
Extraction and partial purification of amylase inhibitors
The seed samples were ground to obtain the fine powder. The powder was suspended in cold sodium phosphate buffer containing 0.02 M NaCl and 0.1 M CaCl2 at pH 6.9. The suspension was then centrifuged (4 ºC) at 3,000 rpm for 10 min, the supernatant was submitted to fractionation by 1 M ammonium sulfate precipitation (20%-60%) and kept overnight at 4 ºC. The precipitate was recovered by centrifugation at 15,000 rpm for 10 min. The pellet was dissolved in 0.02 M sodium phosphate buffer at pH 6.9. The suspension was dialyzed against sodium phosphate buffer overnight at 4ºC. The extract was stored at -20ºC until use.
Protein Quantitation
The protein Quantitation of crude alpha amylase inhibitors was carried out by the method of (Bradford 1976). Bovine serum albumin was taken as standard.
Preparation of Tribolium castaneum (Set I) and Helicoverpa armigera (Set II) extract (amylase source)
The larvae were reared on an artificial diet (Nagarkatti and Satyaprakash 1974). And mid-to-late instar mid guts were isolated via dissection and homogenized with phosphate buffer. The enzyme was extracted out with 0.2 M phosphate buffer pH 6.9. The extract was subjected to centrifugation at 10,000 g for 10 min at 4˚C. The supernatant served as the source of alpha amylase (Ozgur et al., 2008).
Amylase inhibition assay
The reaction sets were prepared in triplicates for two amylase extracts i.e. Set I (T. castaneum) and Set II (H. armigera) and mean values were taken into consideration. 25µl of crude amylase extract and 25µl of enzyme inhibitors extract were mixed and pre incubated at room temperature for 30 min. After the pre-incubation 350µl of 1% starch solution (Prepared in 0.1M Phosphate buffer pH 6.9) was added and kept for incubation for 10 min. After incubation period the reaction was stopped by addition of 300 µl Dinitro Salicylic Acid (DNSA), followed by boiling for 10 min in a water bath. The tubes were cooled and three ml of distill water was added and then absorbance was measured at A540 by UV-Spectrophotometer. The respective control set was prepared with 25µl of distill water instead of amylase inhibitors solution. The inhibitory activity of seed extract was calculated by following formula (Hao et al. 2009).
Inhibitory activity (%) = (A control – A sample) / A control X 100
Enzyme characterization
Amylase from T. castaneum and H. armigera were studied for its activity at various pH and temperature range by setting up the variable physical parameters.
RESULTS
The plant seed extracts showed inhibitory activity against amylase enzyme obtained from T. castaneum and H. armigera respectively (Table-1). The Maize amylase inhibitor (MAI’s) extract showed maximum inhibitory activity against amylase isolated from both i.e. T. castaneum and H.armigera. The amylase inhibitor extracted from Maize shows 82.1 % inhibition against T. castaneum and 85.12% inhibition against H. armigera. The wheat sample showed intermediate activity with 72.12% against T. castaneum and 65.34 % against H. armigera. Whereas amylase inhibitor extracted from the millet and chick pea showed less inhibitory activities as compared to the extracts of Maize and wheat, the inhibitory activity of millet extract was recorded 48.29% against T.castaneum and 43.21% against H.armigera, similarly the second less inhibition was recorded from the extract of chick pea which showed 44.25 % inhibition against T. castaneum and 32.21 % of inhibition against H. armigera.
Protein Quantitation
The quantitative analysis of protein by Bradford method was carried out by keeping Bovine serum albumin (BSA) as standard
Enzyme Characterization
Amylase from T. castaneum and H. armigera showed optimum pH and temperature for hydrolysis of starch at pH 7.4 and 40˚C respectively.
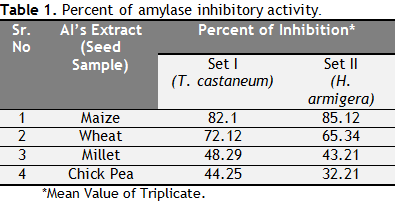 |
Table1 Percent of amylase inhibitory activity. Click here to view full image |
 |
Figure 1 Percent amylase inhition by plant source against T. castaneum and H. armigera. Click here to view full image |
DISCUSSION
The plant is good source of inhibitor as they produce thousands of different compounds in their life span. In agriculture, productivity and quality of product is being loss due to pest. Murthi et al. (2009) reported amylase inhibitors from plant source including T. aestivum against human amylase. In Buonocore et al.,(1977) and Hasenah et al.,(2006) it was reported that T. aestivum and P. amarus exhibited α- amylase inhibitory properties. However Z. mays being an intermittent species of experimentation also showed significant inhibitory properties. The experiments were performed only using hexane extracts by them. However result may be vary with the other solvents like ethyl acetate, ethanol, dichloro methane, etc. can show inhibitory effects for plants which did not prove positive when tested with present one. It could be wise if these sources tested with different solvent against different organism’s amylase.
CONCLUSION
The maize and wheat extracts were found to be more efficient sources of pest amylase inhibitors. The outcomes of the present study is that all four experimental seed samples posses potent insect amylase inhibitors, These inhibitors blocks the activity of insect’s gut amylase with varrying percentage. Such activity is beneficial and can be use to control the pest crop plants in agricultural to ovoid agricultural yield by insect attack.
CONFLICT OF INTEREST
None declared.
REFERENCES
Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 72, 248-254, 1976.
Buonocore V, Petrucci T Silano V. Wheat protein inhibitors of alpha-amylase. Phytochem. 16, 811-820, 1986.
Hao X, Li J, Shi Q, Zhang J, He X, Ma H. Characterization of a Novel Legumin alpha amylase inhibitor from Chickpea (Cicer arietinum L.) seeds. Biosci. Biotechnol. Biochem, 73, 1200-1202, 2009.
Hasenah A, Houghton PJ, Amala S. á-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol, 107, 449-455, 2006.
Kratiger A F. Insect resistance in crops: a case study of Bacillus thuringiensis (Bt) and its transfer to developing countries. ISAAA Briefs 2, 1-42, 1997.
Lawrence PK, Kaundal K R. Plant protease inhibitors in control of phytophagous insects. Elect J Biotech 5, 1-17, 2002.
Murthi PS, Deecaraman M, Kumar KP, Kishanvaidyanat N.Screening of α-amylase inhibitory activities from natural sources. Adv Biotech, 33-36, 2009.
Nagarkatti S, Prakash S. Rearing of Heliothis armigera (Hb.) on artificial diet. Tech. Bull Commonwealth Inst Biol Control, 17, 169-173, 1974.
Ozgur E, Meral Y, Huseyin AO. Identification and characterization of hydrolytic enzymes from the midgut of the cotton bollworm, Helicoverpa armigera Hubner (Lepidoptera: Noctuidae), Turk J Agric, 33, 285-294, 2008









 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.