Himanshu Joshi1, Gururaja MP1, Deepti Joshi2, Shreedevi B3
1NGSM Institute of Pharmaceutical Sciences, Paneer, Deralakhatte, Mangalore-575018, Karnataka, India.
2Expert College, Kodialbail, Mangalore-575003, Karnataka, India.
3KVG Institute of Dental Sciences, Kurunjibag, Sullia, DK-574 327, Karnataka, India.
REVIEW ARTICLE
Volume 2, Issue 2, Page 112-116, May-August 2014.
Article history
Received: 3 August 2014
Revised: 19 August 2014
Accepted: 20 August 2014
Early view: 30 August 2014
*Author for correspondence
E-mail: [email protected]
Mobile/Tel: +91 9482043369
The biomedical waste is the waste that comes from hospitals, nursing homes, clinics, dispensaries, veterinary institutions, animal houses, pathology labs, blood banks etc. which is generated during the diagnosis, treatment or in research activities in the form of animal waste, laboratory waste, human anatomical waste, blood fluids and sharps. Unlike other waste this waste is biohazardous, infectious and pathological in nature, which not only encourages the growth of various pathogens and vectors but also contaminates the non hazardous and non toxic waste, Hence, its handling and disposal becomes an important issue. After the year 1980 the hospital waste was considered as a serious issue, especially HIV and Hepatitis B infected materials which can be a potential risk factor to other patients. This concept of biomedical waste management is new in India; it came into limelight recently after the notification of Bio Medical Waste (BMW) (Management and Handling) Rules 1998. The present paper highlights the various issues concerned with the biomedical waste and the latest updates, with an objective to create awareness regarding the handling and management of biomedical waste among biomedical researchers.
Keywords: biomedical waste, handling and disposal, waste management.
INTRODUCTION
Bio-medical waste is hazardous and toxic in nature because of its high potential of transmission of diseases not only for the rag pickers and waste workers but also for the general public. Bio-medical waste constitutes an estimated 1.5 to 2 per cent of municipal waste in the urban areas of which around three fourths of waste is non hazardous and non toxic and there is a need to segregate the rest hazardous and toxic waste at the source itself (Times of India, 2014). Such waste must be handled and managed properly as per the rules. In this connection, the Bio-Medical Waste Rules-1998 (BMW Rules, 1998) have been in place for the past years but because of various challenges in the implementation of the system like, lack of capacity among generators and regulators, inadequate infrastructure and finance, they could not solve the purpose. On 21st September 2011, (BMW Rules, 2011) the Ministry of Environment & Forests notified the new draft Bio-Medical Waste (Management & Handling) Rules, 2011 under the Environment (Protection) Act, 1986 to replace the earlier Rules (1998) and the amendments thereof (Rao, 2004; Shalini, 2008; Vanesh, 2011; Singh, 2007; Cseindia, 2014).
 |
Figure 1. Source of Biomedical waste(BMW) Click here to view full image |
Source of biomedical waste (BMW) (special features)
2011 comprises of 17 rules along with 6 schedules and 6 application forms formats (BMW The Bio Medical Waste (Management & Handling) Rules, Rules, 2011).
 |
Table 1. Various rules under BMW (M&H) rules 2011. Click here to view full image |
 |
Table 2. Various schedules under BMW (M&H) rules 2011. Click here to view full image |
 |
Table 3. Various application forms under BMW (M & H) rules 2011. Click here to view full image |
Modification of rules 2011 over rules 1998 (cseindia.org)
- As per rules 2011, every occupier generating Bio-medical waste (BMW), irrespective of the quantum of waste is subjected under the BMW Rules and requires to obtain authorization whereas as per rules 1998, occupiers with more than 1000 beds were required to obtain authorization.
- Duties of the operator did not form a part of the rules in 1998. While they are listed in rules of 2011.
- The number of categories of Biomedical Waste was ten in 1998 which is now eight in rules of 2011.
- Form VI is added to the rules 2011 (the report of the operator on health care establishment is (HCEs) not handing over the BMW added to the Rules)
- A format for annual report is also introduced in the Rules 2011.
Applicability of rules
To whom these rules are applicable?
As per the latest Bio- Medical Waste (Management and Handling) rules 2011, it is applicable to all who generate, collect, receive, store, transport, treat, dispose, or handle bio- medical waste in any form.
To whom these rules are not applicable?
- a) Radioactive wastes covered under provision of the atomic energy act 1962 and the rules made there under.
- b) Hazardous chemicals covered under manufacture, storage and import of Hazardous chemicals rules 1989 made under Environment (Protection) Act 1986
- c) Municipal solid waste covered under the (Management and Handling) rules 2000
- d) Lead acid batteries covered under Batteries (Management and Handling) rules 2001
- e) Hazardous waste covered under the Hazardous waste (Management, Handling and Transboundary Movement) rules 2008 made under Act.
Terms defined under the bio- medical waste (management and handling) rules 2011.
Animal house: “Animal House” means a place where the animals are reared/ kept for the purpose of experiments or testing:
Authorization:“Authorization” means permission granted by the prescribed authority for the generation, collection, reception, storage, transportation, treatment, disposal and/ or any other form of handling of bio-medical waste in accordance with these rules and guidelines issued by the Central Pollution Control Board, Ministry of Environment and Forests, Ministry of Health and Family Welfare, Government of India.
Authorized Person: “Authorized Person” means an occupier or operator authorized by the prescribed authority to generate, collect, store, transport, treat, dispose, and/ or handle bio-medical waste in accordance with these rules and any guidelines issued by the Central Pollution Control Board, Ministry of Environment and Forests, Ministry of Health and Family Welfare, Government of India.
Bio- Medical Waste “Bio- Medical waste” means any waste, which is generated during the diagnosis, treatment or immunization of human beings or animals or in research activities pertaining thereto or in the production or testing of biologicals, including categories mentioned in schedule I of these rules.
Biologicals“Biologicals” means any preparation made from organism or micro-organisms or product of metabolism and biochemical reactions intended for use in diagnosis, immunization or the treatment of human beings or animals or in research activities pertaining thereto.
Bio- Medical Waste treatment and disposal facility: “Bio- Medical Waste treatment and disposal facility” means any facility wherein treatment disposal of bio-medical waste or processes incidental to such treatment and or disposal is carried out and includes common treatment facilities.
Occupier: “Occupier” means a person having administrative control over the institution and the premises generating Bio-Medical waste, which includes a hospital, nursing home, clinic, dispensary, veterinary institution, animal house, pathological laboratory, blood bank, health care facility and clinical establishment by whatever name it may be called.
Operator of a common biomedical waste treatment facility: “Occupier of a common biomedical waste treatment facility” means a person who owns or controls or operates a common facility for the collection, reception, storage, transport, treatment, disposal or any form of handling of biomedical waste.
Duties of occupier and operatorOccupier means i.e Institutions, animal houses, hospitals, laboratories etc, who generates the biomedical waste. The following duties have been assigned to the occupiers in the Bio-Medical Waste (Management & Handling) Rules, 2011 under the Environment (Protection) Act, 1986.
Occupier: As per the Bio-Medical Waste (Management & Handling) Rules, 2011 under the Environment (Protection) Act, 1986 following duties have been assigned to Occupier (generating bio-medical waste):
1. It is a responsibility of a occupier that the bio-medical waste should be handled without any adverse effect to human health and environment.
2. He should provide necessary training to the people who are involved in the handling of bio-medical waste.
3. Immunization of the people who are involved in the handling of bio-medical waste.
4. Segregation of bio-medical waste at the time of generation.
5. He should provide occupational safety to all the people who are involved in the handling of bio-medical waste and he should provide appropriate and adequate personal protective equipments.
6. He should conduct health checkup for all the people who are involved in the handling of bio-medical waste and maintain the register for the same.
7. It is a responsibility of a occupier to maintain and update day by day bio-medical waste management register.
8. He should develop a proper system to report unintended accidents (i.e Sharp injuries, mercury spills) or fire hazards which may occur in the handling of bio-medical waste.
9. Establish a bio-medical waste management cell.
Operator: For the very first time in the Bio-Medical Waste (Management & Handling) Rules, 2011 following duties have been assigned to Operator:
1. Operator has to take all necessary steps to ensure that the bio medical waste collected from the occupier (bio medical waste generator) is transported, handled, stored, treated and disposed properly without any adverse effect to human health and environment.
2. He should ensure timely collection of bio medical waste.
3. He should conduct training program regarding the handling of bio medical waste for all the workers involved in handling of such waste.
4. He should ensure the immunization of all the people involved in the handling of bio medical waste and maintain the records for the same.
5. He should provide occupational safety to all the people who are involved in the handling of bio-medical waste and provide appropriate and adequate personal protective equipments.
6. He should develop a proper system to report unintended accidents (i.e. Sharp injuries, mercury spills) or fire hazards which may occur in the handling of bio-medical waste along with remedial action taken to solve such problems and to record and update such information in form 3.
7. He should maintain a log book for each of its treatment equipment according to categories of waste treated; weight of batch; date, time and duration of treatment cycle and total hours of operation.
He should ensure that the prescribed authorities are immediately informed regarding the Health Care Establishments or Health Care Facilities, which are not handing over the segregated bio medical waste in accordance with the rules prescribed in form 4.
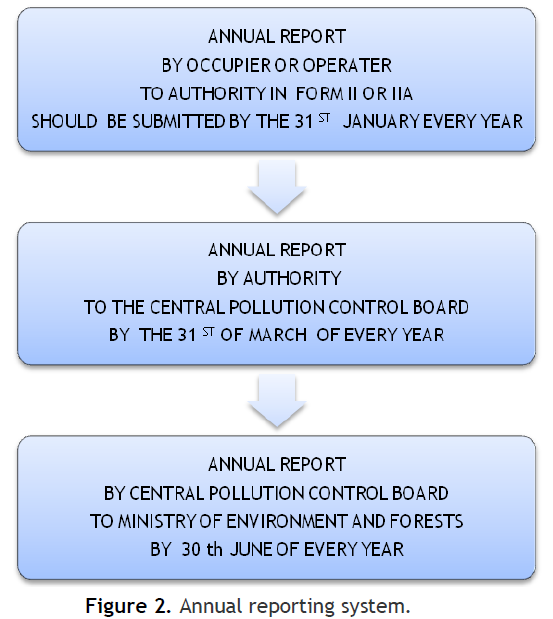 |
Figure 2. Annual reporting system. Click here to view full image |
 |
Figure 3. . Colour coding for different category of bio-medical waste. Click here to view full image |
Accident reporting. Biomedical waste management is a multistep procedure which involves collection, storage, transportation and disposal. Any accident during the following processes has to be reported in a prescribed format as per the rules, in Form No III. These accidents may take place at institution, hospital, or any place producing biomedical waste.
Labeling requirement: As per the recent rules regarding the collection and disposal of biomedical waste, Biohazard symbol has to be affixed on the containers or bags used for the collection and disposal of biomedical waste with their respective colours (Table 4, 5). The mark on the containers or bags should be permanent, non-washable and prominently visible.
 |
Table 4. .Color coding and Type of container for disposal of BMW. Click here to view full image |
 |
Table 5. . Color coding and type of Container for Disposal of BMW. Click here to view full image |
CONCLUSION
This paper aims to emphasize on the responsibility of not only the people involved in BMW or biomedical scientists but also all the stakeholders in minimizing and managing the biomedical waste as per the guidelines. The need of the hour is to address such serious issue not only by social responsibility but also by awareness, education, training, strong policy making and legislative framework. Proper Biomedical waste management (BMW) (which includes its differentiation, segregation, placing in different containers, colour coding and numbering, storage or temporary holding, transportation and disposal) is the only way to deal with the toxic hazardous waste material and reduce its implications for human and environmental health.
REFERENCES
BMW Rules; Biomedical waste (management and Handling) Rules 1998. Ministry of Environment and Forests Notification, Government of India- http://envfor.nic.in/legis/hsm/biomed.html
BMW Rules; Biomedical waste (management and handling) Rules 2011. Ministry of Environment and Forests Notification, Government of India.–
http://moef.nic.in/downloads/public-information/salient-features-draft-bmwmh.pdf
Cseindia; Center for Science and Environment, India, 2014.- www.cseindia.org/node/3702
Rao SK, Ranyal RK, Bhatia SS, Sharma VR. Biomedical waste management: An Infra structural Survey of hospitals. MJAFI. 60, 371-382, 2004.
Shalini S, Chauchan SV. Assessment of bio-medical waste management in three apex government hospital of Agra. J Environ Biol. 29, 159-162, 2008.
Singh VP, Biswas G, Sharma JJ. Biomedical waste management –An Emerging Concern in Indian Hospitals. Indian J Forensic Med Toxicol. 1, 7-12, 2007.
Times of India, 2014- http://timesofindia.indiatimes.com/topic/Bio-Medical-Waste.
Vanesh M, Dwivedi S, Hassan MA, Misra RP. Knowledge attitude & practices about biomedical waste management among health care personnel: A cross sectional study. Indian J Community Med. 36, 143-145, 2011.








 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.